I dont know why I'm having such a hard time applying molality to questions.. I just can't seem to get any of them..
I understand that molality = moles solute/kg solution and solution = solute + solvent. But some things just don't ever seem to work out...
Like this Kaplan question: How many grams of Al2(SO4)3 are needed to make 87.5 g of 0.3 m Al2(SO4)3 solution?
Answer:
This rather tricky question on concentration units and solution stoichiometry requires that you apply the definition of molality, or moles of solute per kilogram solvent, although a glance at the answer choices indicates that full calculation is unnecessary. We can calculate the formula weight of aluminum sulfate as 342 g/mol (2 x 27 + 3 x 32 + 12 x 16, or 54 + 3 x 96) and thus deduce that 102.6 grams are required per kilogram of solvent (0.3 x 342 = 102.6). The total mass of a solution produced by dissolving 102.6 g of aluminum sulfate in one kilogram, i.e., 1000 g, of solvent will then be 1102.6 grams. We can set up and rearrange a ratio now, to find the quantity of Al2(SO4)3 required for the 87.5 g of solution given in the question:
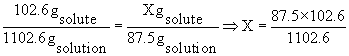
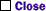
That is the explanation. What I don't get about the explanation is that if the molality was given as 0.3 m. Then how could they say that 102.6g is needed for each 1000g of solvent, but then use 1102.6g of solvent in the calculation for the answer.. that doesn't make sense to me. Shouldn't the amount of solute be 1000-102.6g??
Ahhh I'm so confused.
I understand that molality = moles solute/kg solution and solution = solute + solvent. But some things just don't ever seem to work out...
Like this Kaplan question: How many grams of Al2(SO4)3 are needed to make 87.5 g of 0.3 m Al2(SO4)3 solution?
Answer:
This rather tricky question on concentration units and solution stoichiometry requires that you apply the definition of molality, or moles of solute per kilogram solvent, although a glance at the answer choices indicates that full calculation is unnecessary. We can calculate the formula weight of aluminum sulfate as 342 g/mol (2 x 27 + 3 x 32 + 12 x 16, or 54 + 3 x 96) and thus deduce that 102.6 grams are required per kilogram of solvent (0.3 x 342 = 102.6). The total mass of a solution produced by dissolving 102.6 g of aluminum sulfate in one kilogram, i.e., 1000 g, of solvent will then be 1102.6 grams. We can set up and rearrange a ratio now, to find the quantity of Al2(SO4)3 required for the 87.5 g of solution given in the question:
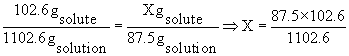
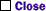
That is the explanation. What I don't get about the explanation is that if the molality was given as 0.3 m. Then how could they say that 102.6g is needed for each 1000g of solvent, but then use 1102.6g of solvent in the calculation for the answer.. that doesn't make sense to me. Shouldn't the amount of solute be 1000-102.6g??
Ahhh I'm so confused.
