When you have sp3 hybridization, you fill one electron in each orbital right, thus you have 4 unpaired orbitals for bonding.
But with sp2 hybridization, you have an empty p orbital that is higher in Energy than the 3 sp2 hybrid orbitals...
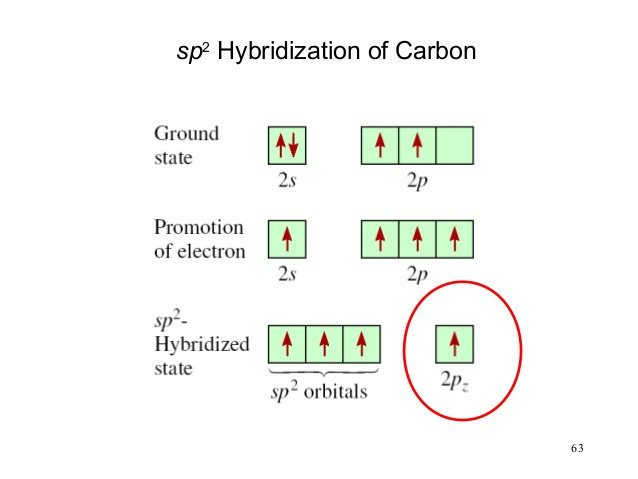
so now to my question: when such an atom is in a sp2 bond, is the p orbital always unpaired?
Isn't it true that (at least one form of) resonance requires adjacent p orbitals to be unpaired?
Yet how can a double-bonded atom be able to have this resonance if that means it's p-orbital must be overlapping with the adjacent atoms p-orbital (in order to be a double bond...) thus making it actually unavailable for resonance?
For example, carbon of benzene are all in a conjugated pi system (all Carbons are sp2) meaning they all have an unpaired p orbital? so how can there be double bonds between the carbons if that would imply that the p-orbitals are not available for resonance?
Or is it that aromatic compounds double bonds are not truly double bonds thus the p orbital is actually unpaired?
..thanks for your time
But with sp2 hybridization, you have an empty p orbital that is higher in Energy than the 3 sp2 hybrid orbitals...
so now to my question: when such an atom is in a sp2 bond, is the p orbital always unpaired?
Isn't it true that (at least one form of) resonance requires adjacent p orbitals to be unpaired?
Yet how can a double-bonded atom be able to have this resonance if that means it's p-orbital must be overlapping with the adjacent atoms p-orbital (in order to be a double bond...) thus making it actually unavailable for resonance?
For example, carbon of benzene are all in a conjugated pi system (all Carbons are sp2) meaning they all have an unpaired p orbital? so how can there be double bonds between the carbons if that would imply that the p-orbitals are not available for resonance?
Or is it that aromatic compounds double bonds are not truly double bonds thus the p orbital is actually unpaired?
..thanks for your time
