- Joined
- May 17, 2008
- Messages
- 3,023
- Reaction score
- 3,619
I have a question on the following problem (from Qvault):
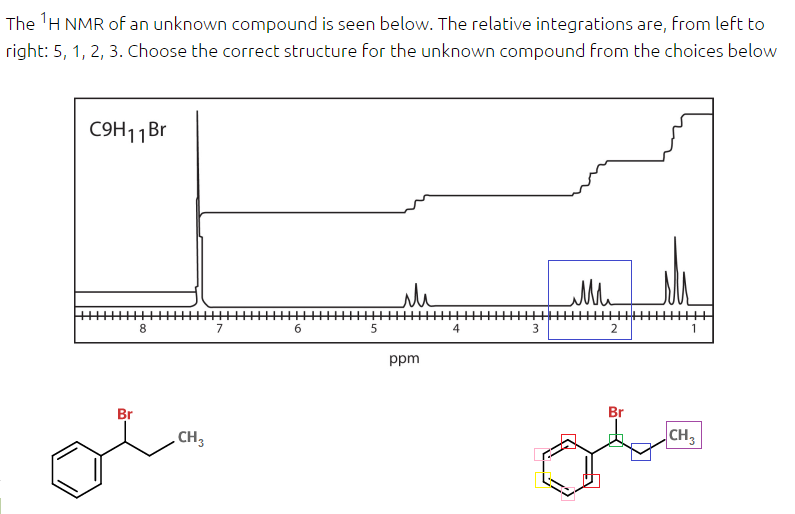
The answer is the figure drawn on the bottom left. There's two issues I have:
1. Why are there only 4 H-environments shown? I tried selecting all the ones I could find (color coded to distinguish them in the bottom right) and I'm getting 7 total. It seems like they just lumped all the protons from the benzene ring as one environment of H's. Is this standard for proton NMR's as they're given on the DAT or would the protons of a benzene ring (assuming they're not symmetrical) be distinguishable?
2. The peaks on the graph surrounded by the blue rectangle correspond to the same color carbon in the figure on the bottom right, according to the answer key. But from what I can tell there should be 8 peaks present because it has a neighbor (purple rectangle) with 3 H's which gives us a quartet and a neighbor with 1 H (green rectangle) which means a quartet of a doublet. Is this just supposed to represent a multiplet and the actual peak count doesn't matter? At what point do we start disregarding the number of peaks and assume it is just a multiplet?

The answer is the figure drawn on the bottom left. There's two issues I have:
1. Why are there only 4 H-environments shown? I tried selecting all the ones I could find (color coded to distinguish them in the bottom right) and I'm getting 7 total. It seems like they just lumped all the protons from the benzene ring as one environment of H's. Is this standard for proton NMR's as they're given on the DAT or would the protons of a benzene ring (assuming they're not symmetrical) be distinguishable?
2. The peaks on the graph surrounded by the blue rectangle correspond to the same color carbon in the figure on the bottom right, according to the answer key. But from what I can tell there should be 8 peaks present because it has a neighbor (purple rectangle) with 3 H's which gives us a quartet and a neighbor with 1 H (green rectangle) which means a quartet of a doublet. Is this just supposed to represent a multiplet and the actual peak count doesn't matter? At what point do we start disregarding the number of peaks and assume it is just a multiplet?
Last edited:
