Hello to the respected forum of anesthesia..
Had a case recently and I was thinking about it. Pt was 80 y.o. Male with preexisting HTN on ACE-I, mild dementia, overweight ( 100kg at around 1,80) and pertinent surgical Hx of repaired ing hernia 2 years prior. Had no bowel movement over 3 days and was posted for exlap for possible mechanical SBO. Labs were wnl, except Hb which was 9, Cr of 4.0, BUN of 100 ( prerenal AKI? )
On presentation he was in apparent distress, looking septic HR 110 in Afib, BP 170/90, Spo2 95% on Venturi 50%, tachypneic with a hugeee abdomen. Anyways we put an aline, induce, CVC and surgery proceeds. I started some fluids and give around 3L for presumed deficit then I continued at 1-2ml/kg/hr. Oxygenation remained acceptable at an FiO2 55%, PEEP of 8, Peaks around 30-35 which subsided to 23-25 after abdomen was opened. Urine output was at ~500cc for the first hr Patient was stable with HR in 80's ( Afib so no PPV, SPV guidance), normal MAP's around 90. Surgery went on because apparently pt had a tumor in right colon that had caused a hole in the colon with spillage of bowel content so they had to resect it and they did an anastomosis( no colostomy?). Anyway I was alone in the first hour, then my attending came back and told me why was I withholding the fluids, that the patient was profoundly hypovolemic due to ileus so we started giving more LR+NS . At around the 6L mark patient started to look worse with norepi requirement and a little worsening oxygenation. I think we gave 2 more liters after that, and the surgery was coming to a close. Patient now had a 2.5-3ug/kg/min norepi req + bumps of epi to sustain MAP>65. Oxygenation was PaO2 of 90 at 70% FiO2 with the same PEEP.
Of course we didn't extubate and my attending considered the pt terminal due to the huge pressor requirement. Anyways I shifted the pt to ICU and called it a night since it was 6am.
48 hrs later I went to check on the pt and to my big surprise he is extubated on a venturi 40% with minimal norepi req. I asked the fellow about mgmt, echo?. He said they gave some more fluids but also put the pt on CVVHD for worsening hyperkalemia and reduced urine output and of course gave broad spectrum Abx. No echo done yet ( pt only got a single dose of ciprofloxacin in the ED ). Then I lost the pt since I was rotating at a different hospital.
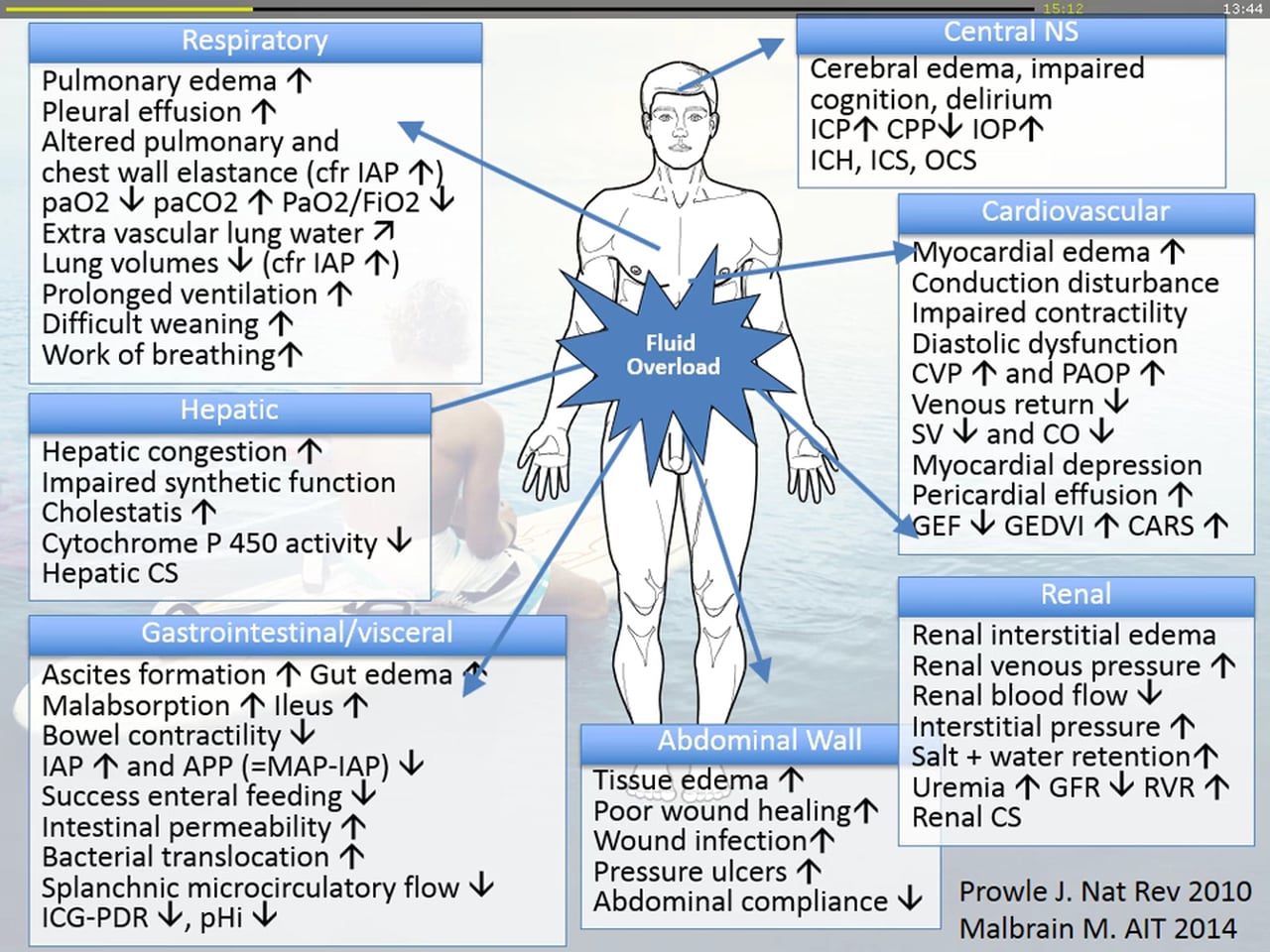
I am having a thought and so far none of the answers of my attendings satisfied my curiosity. So, we all know that fluid management should be a thoughtful process and overload can have deleterious effects on ileus, LOS, wound healing etc, but what I wanted to know is : Is it possible to cause iatrogenic cardiogenic shock with fluid administration in a patient like this with possible untreated diastolic heart failure, but also in a patient without any hint of heart failure? Or was the deterioration due to sepsis that was improved with proper Abx? Actual hypovolemia that needed more fluids?
Thanks in advance for reading the post. Any wisdom is really appreciated!
Had a case recently and I was thinking about it. Pt was 80 y.o. Male with preexisting HTN on ACE-I, mild dementia, overweight ( 100kg at around 1,80) and pertinent surgical Hx of repaired ing hernia 2 years prior. Had no bowel movement over 3 days and was posted for exlap for possible mechanical SBO. Labs were wnl, except Hb which was 9, Cr of 4.0, BUN of 100 ( prerenal AKI? )
On presentation he was in apparent distress, looking septic HR 110 in Afib, BP 170/90, Spo2 95% on Venturi 50%, tachypneic with a hugeee abdomen. Anyways we put an aline, induce, CVC and surgery proceeds. I started some fluids and give around 3L for presumed deficit then I continued at 1-2ml/kg/hr. Oxygenation remained acceptable at an FiO2 55%, PEEP of 8, Peaks around 30-35 which subsided to 23-25 after abdomen was opened. Urine output was at ~500cc for the first hr Patient was stable with HR in 80's ( Afib so no PPV, SPV guidance), normal MAP's around 90. Surgery went on because apparently pt had a tumor in right colon that had caused a hole in the colon with spillage of bowel content so they had to resect it and they did an anastomosis( no colostomy?). Anyway I was alone in the first hour, then my attending came back and told me why was I withholding the fluids, that the patient was profoundly hypovolemic due to ileus so we started giving more LR+NS . At around the 6L mark patient started to look worse with norepi requirement and a little worsening oxygenation. I think we gave 2 more liters after that, and the surgery was coming to a close. Patient now had a 2.5-3ug/kg/min norepi req + bumps of epi to sustain MAP>65. Oxygenation was PaO2 of 90 at 70% FiO2 with the same PEEP.
Of course we didn't extubate and my attending considered the pt terminal due to the huge pressor requirement. Anyways I shifted the pt to ICU and called it a night since it was 6am.
48 hrs later I went to check on the pt and to my big surprise he is extubated on a venturi 40% with minimal norepi req. I asked the fellow about mgmt, echo?. He said they gave some more fluids but also put the pt on CVVHD for worsening hyperkalemia and reduced urine output and of course gave broad spectrum Abx. No echo done yet ( pt only got a single dose of ciprofloxacin in the ED ). Then I lost the pt since I was rotating at a different hospital.
I am having a thought and so far none of the answers of my attendings satisfied my curiosity. So, we all know that fluid management should be a thoughtful process and overload can have deleterious effects on ileus, LOS, wound healing etc, but what I wanted to know is : Is it possible to cause iatrogenic cardiogenic shock with fluid administration in a patient like this with possible untreated diastolic heart failure, but also in a patient without any hint of heart failure? Or was the deterioration due to sepsis that was improved with proper Abx? Actual hypovolemia that needed more fluids?
Thanks in advance for reading the post. Any wisdom is really appreciated!