- Joined
- Dec 26, 2006
- Messages
- 7,100
- Reaction score
- 17,534
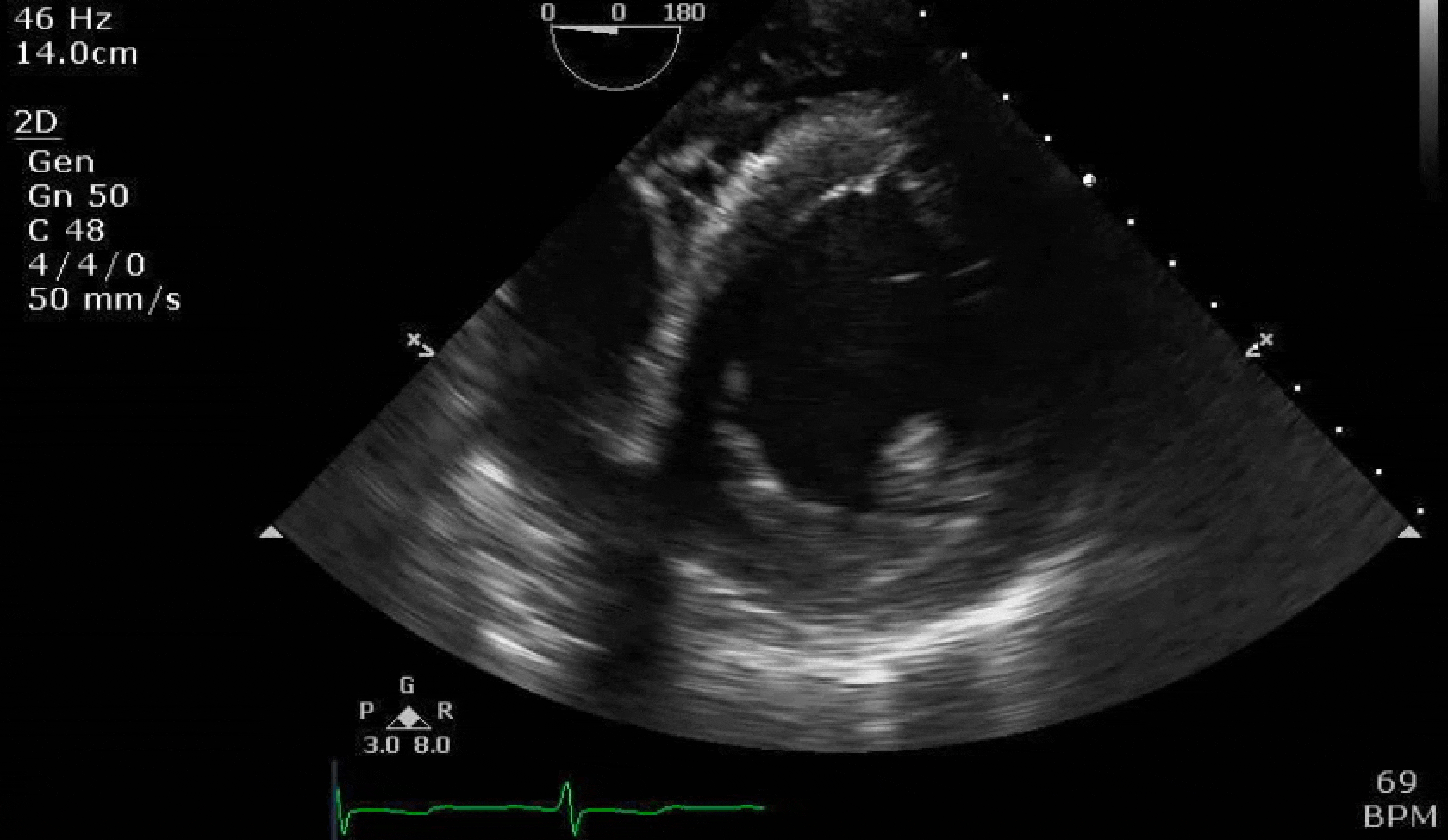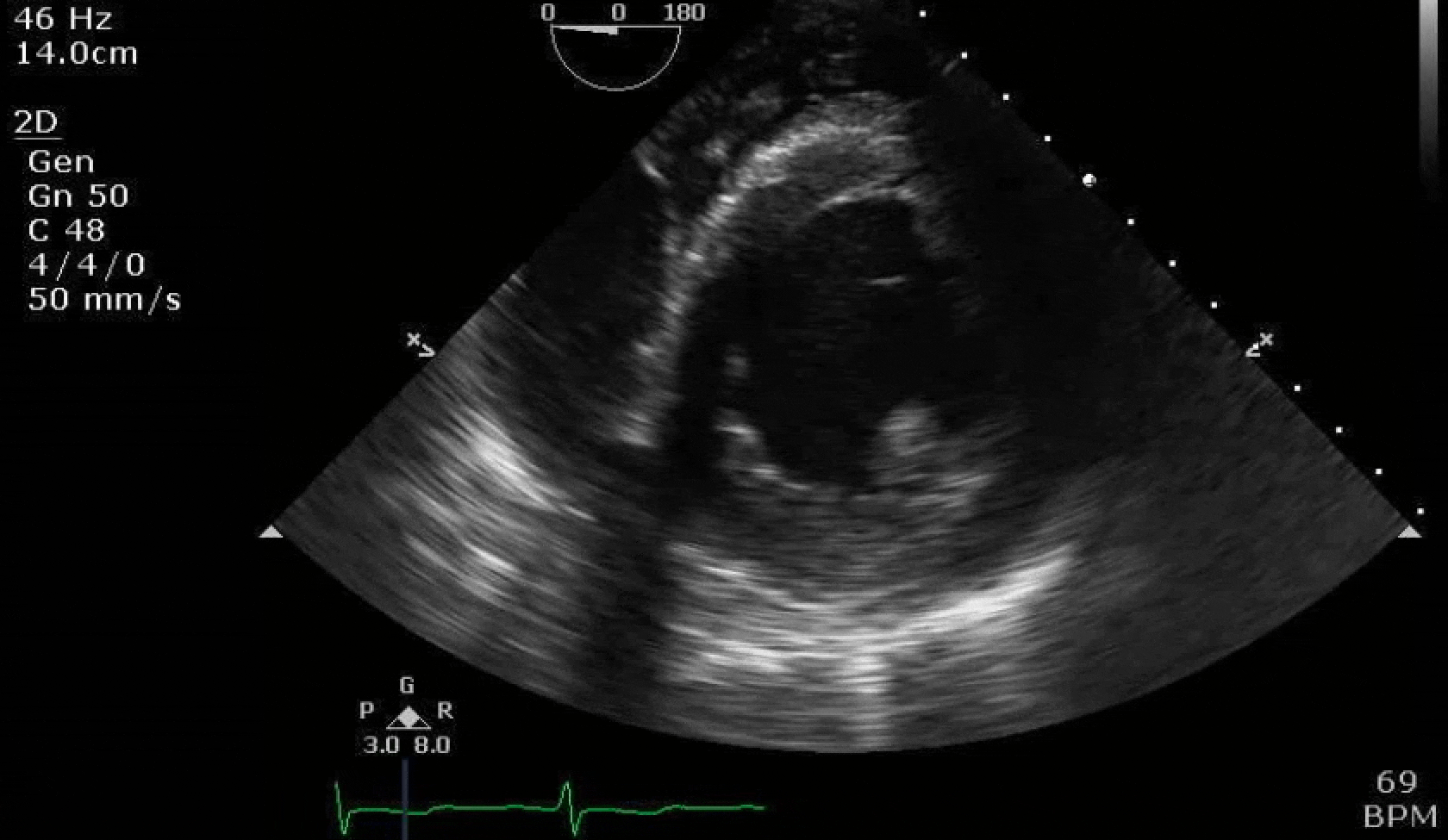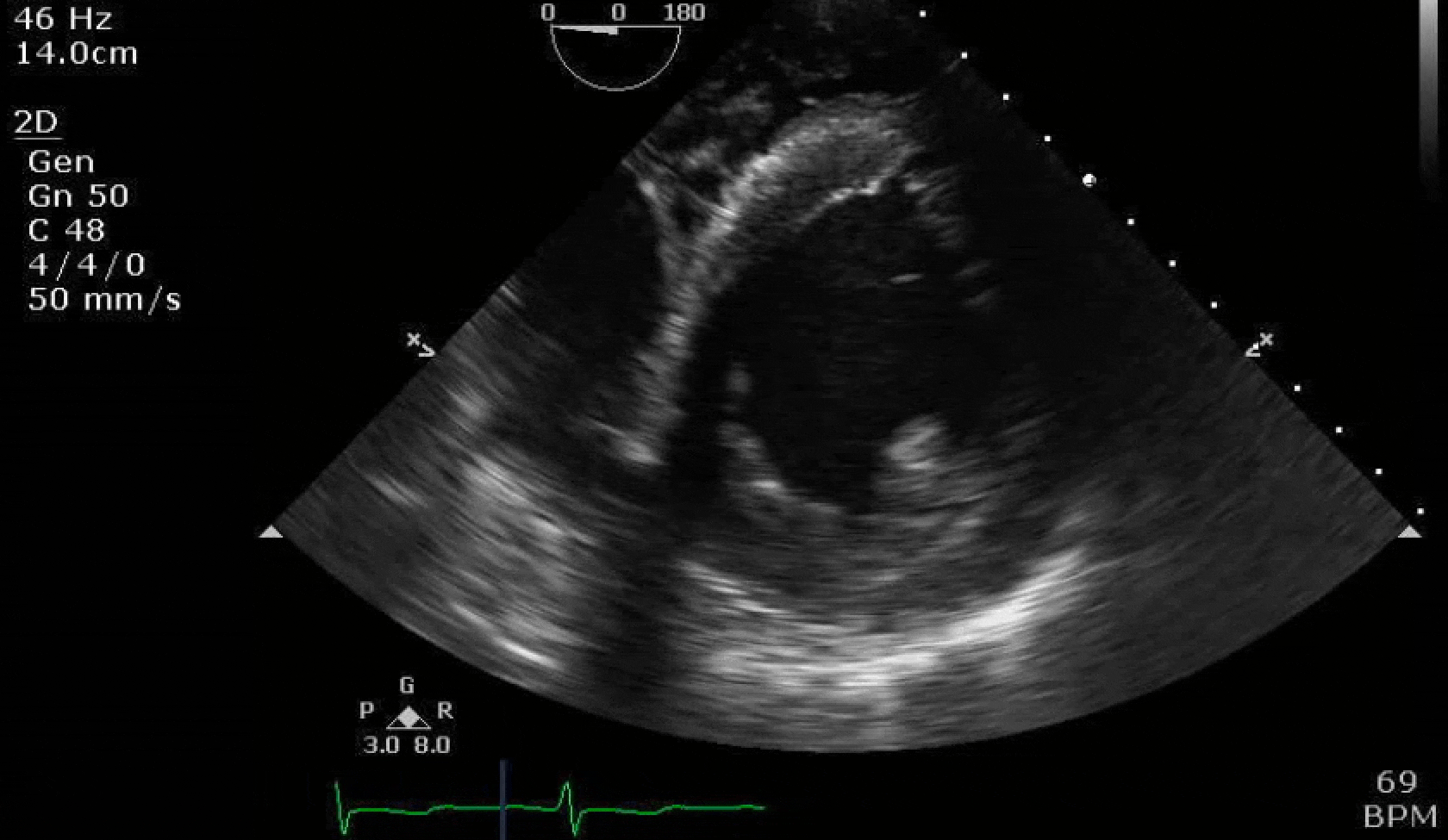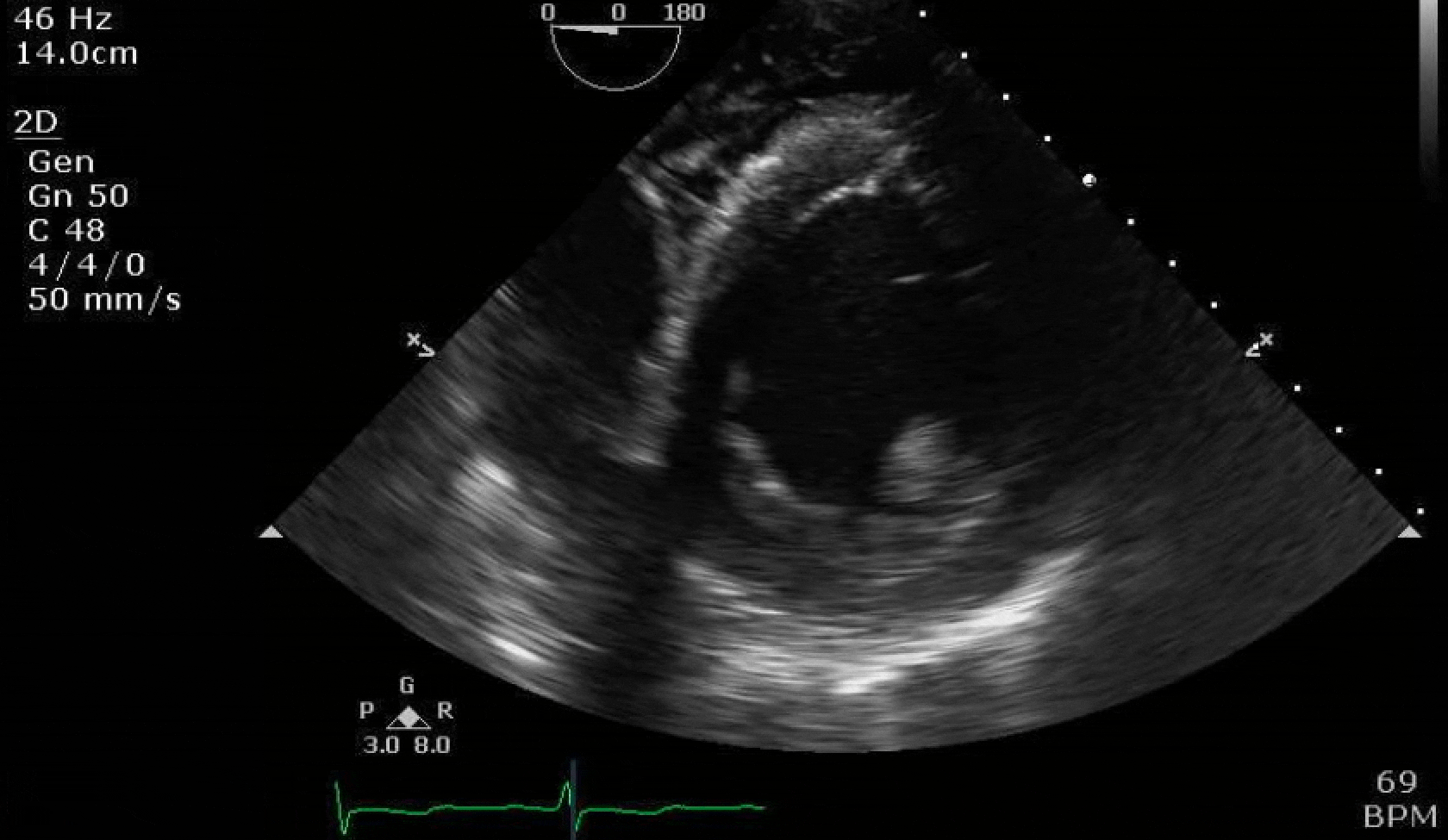
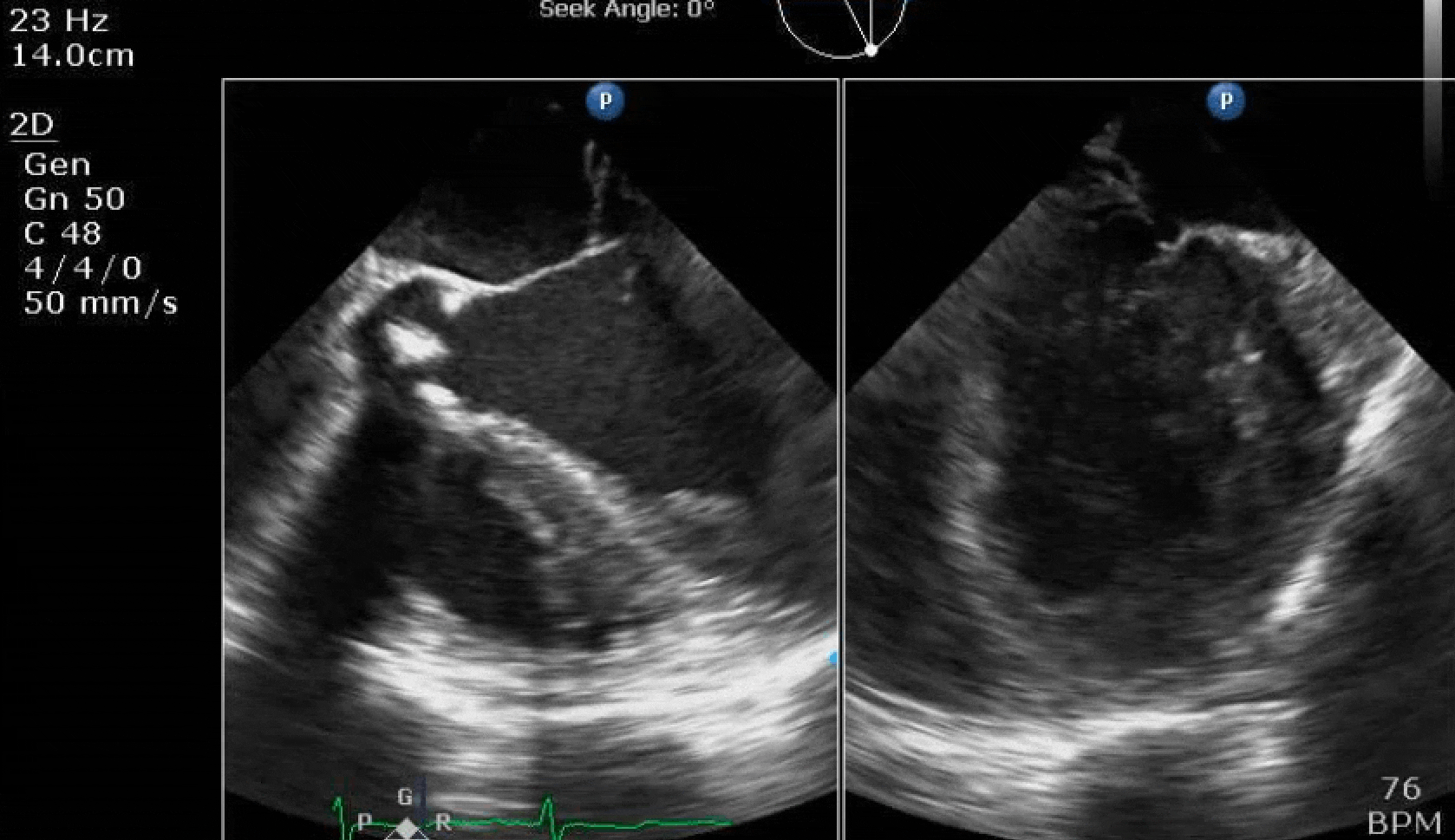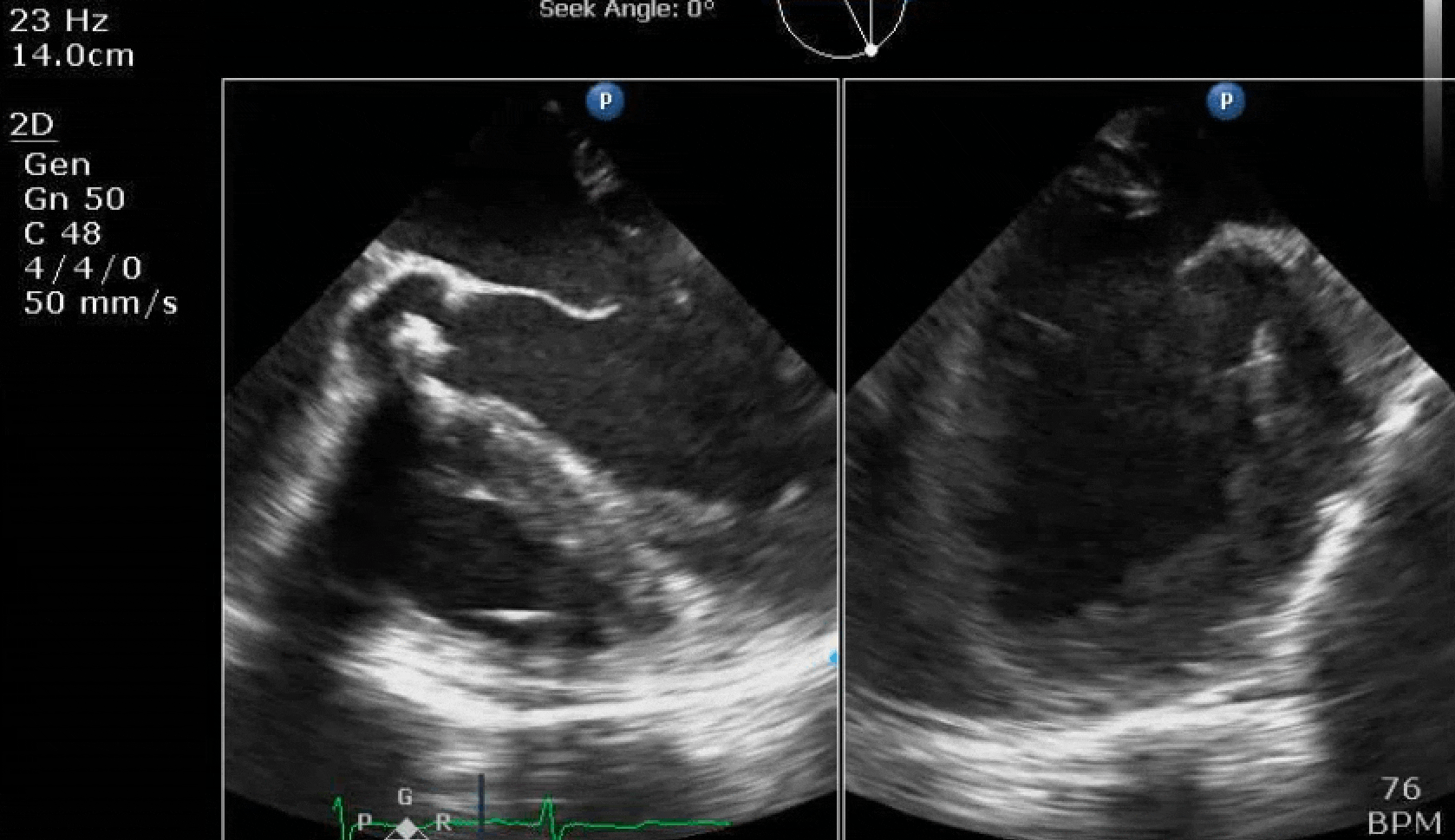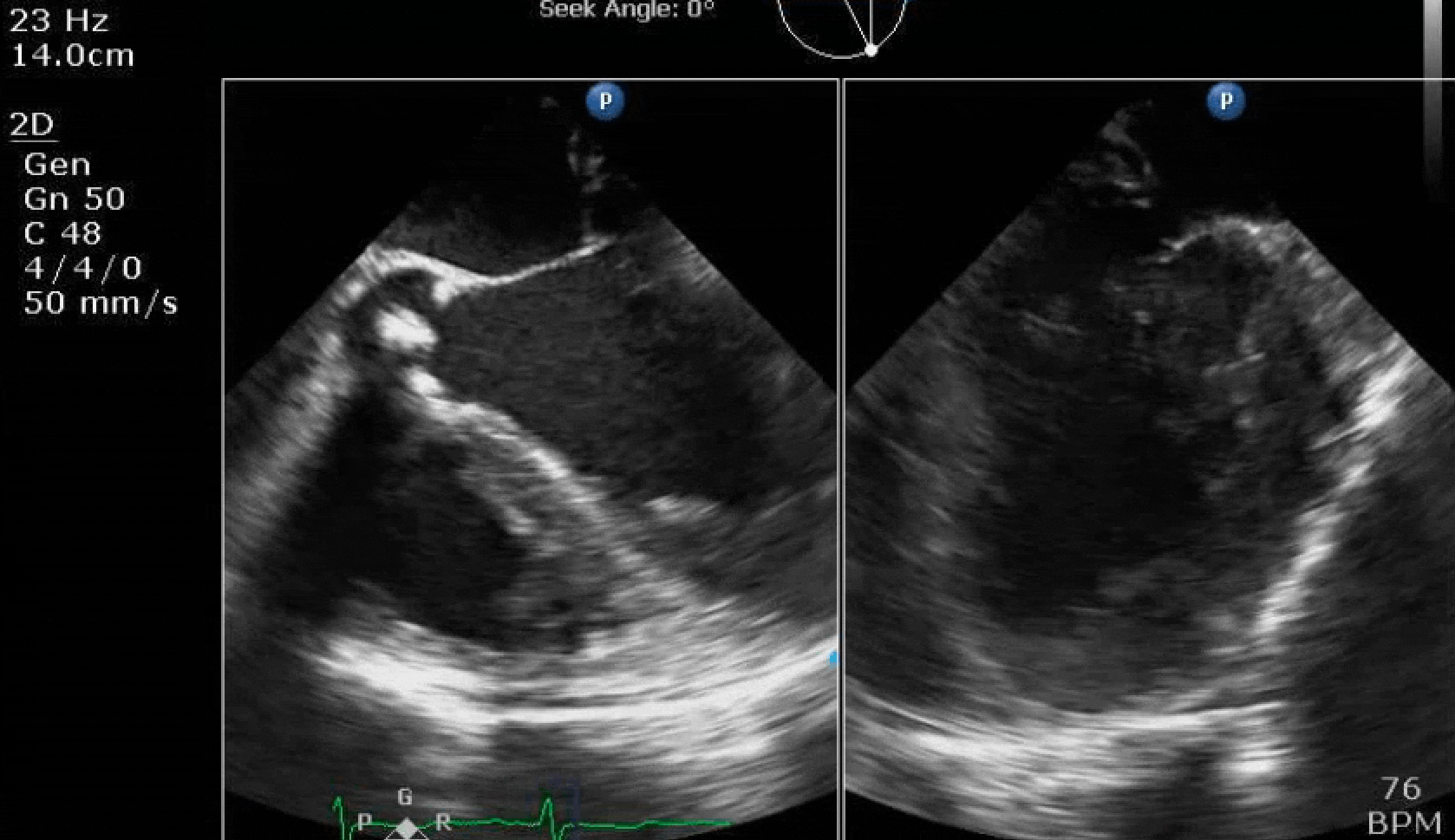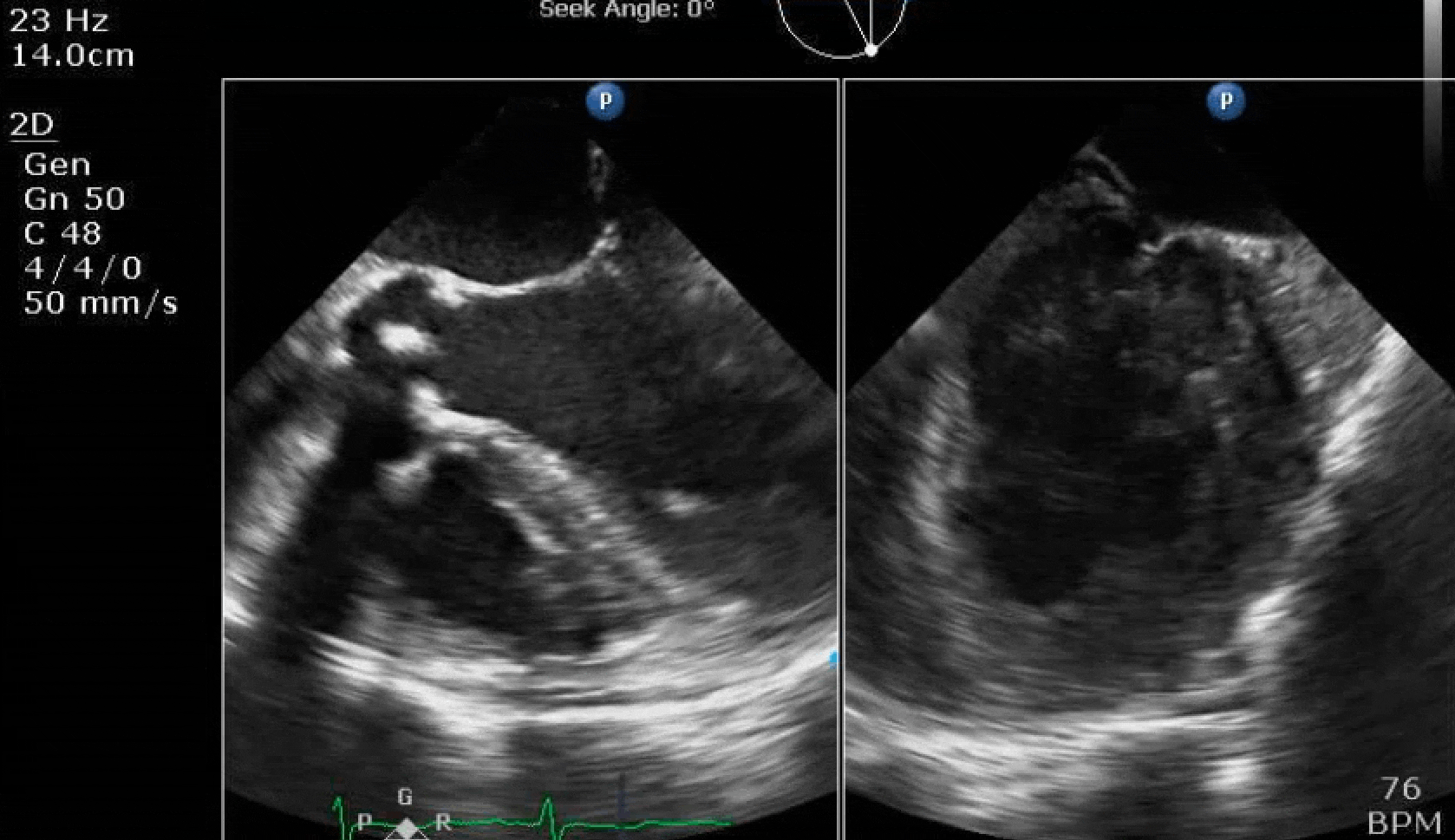
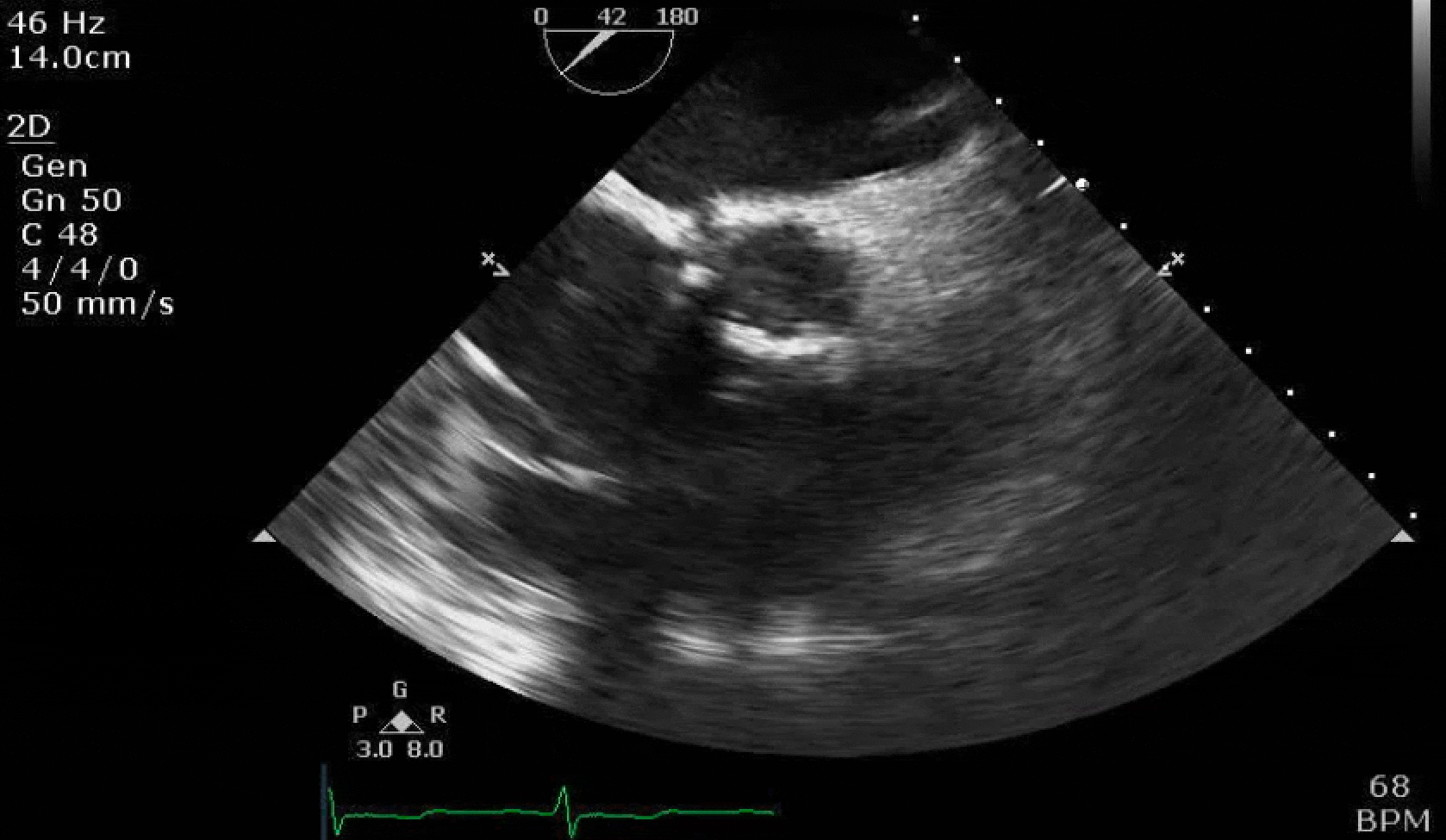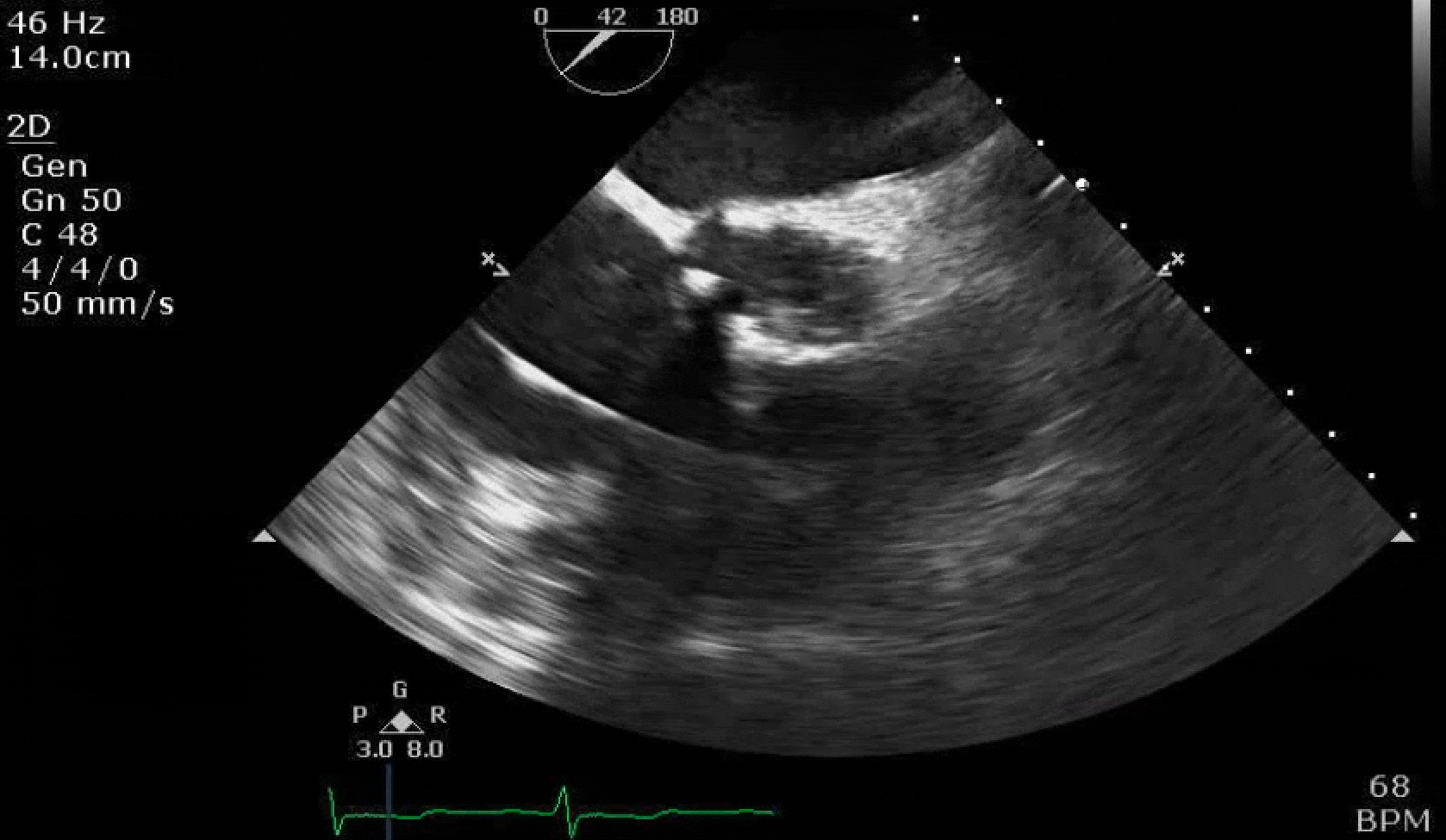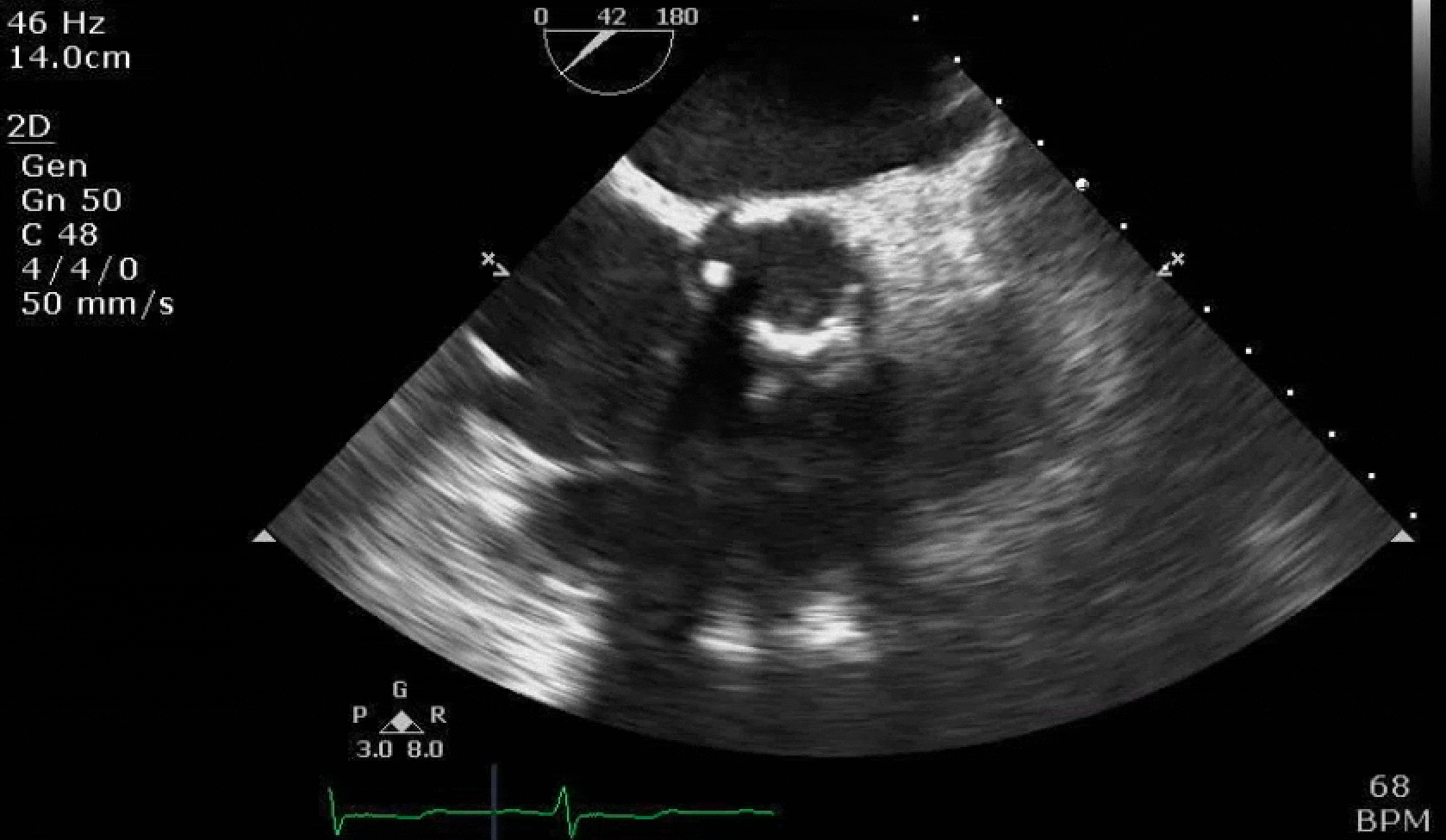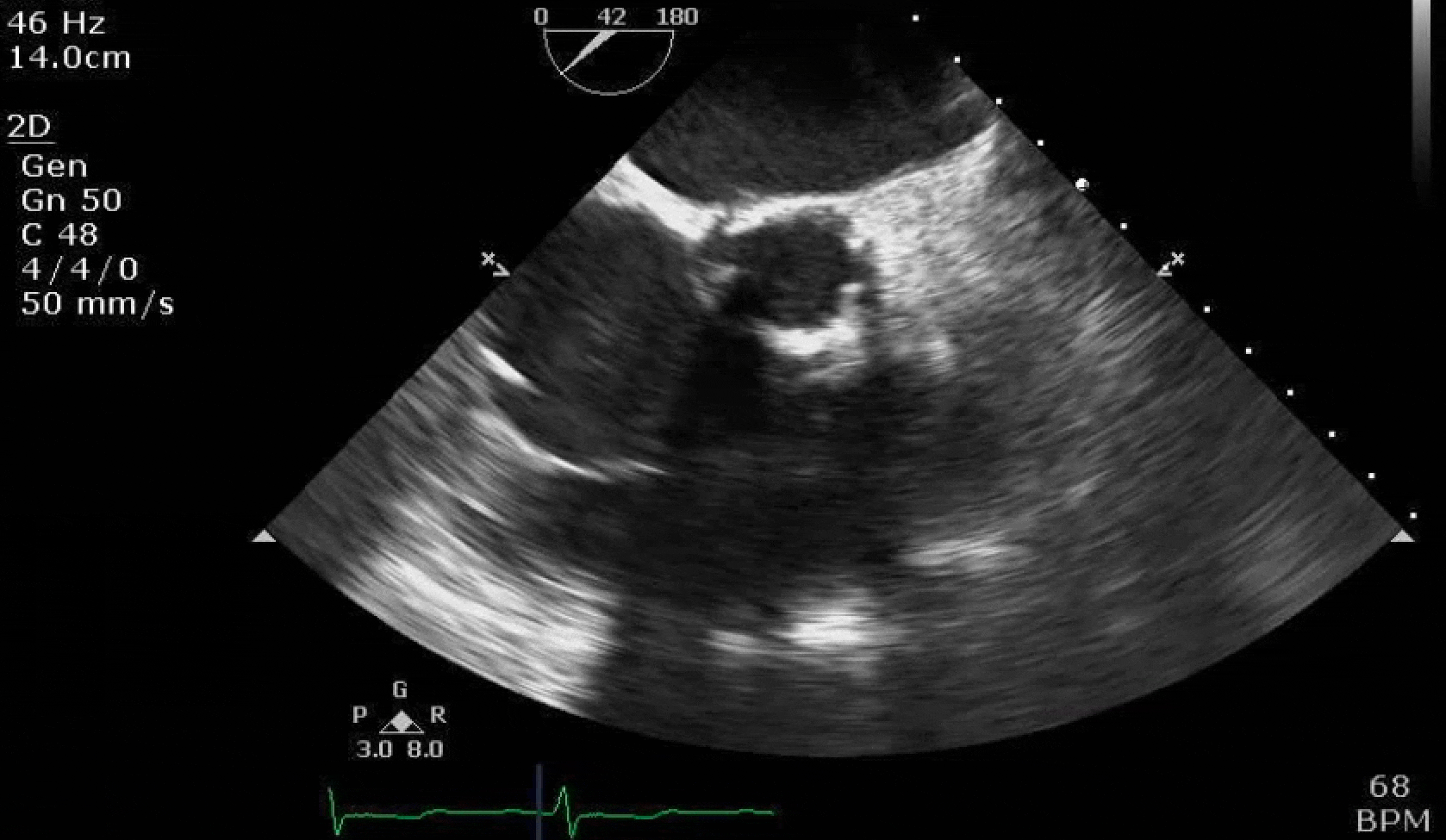
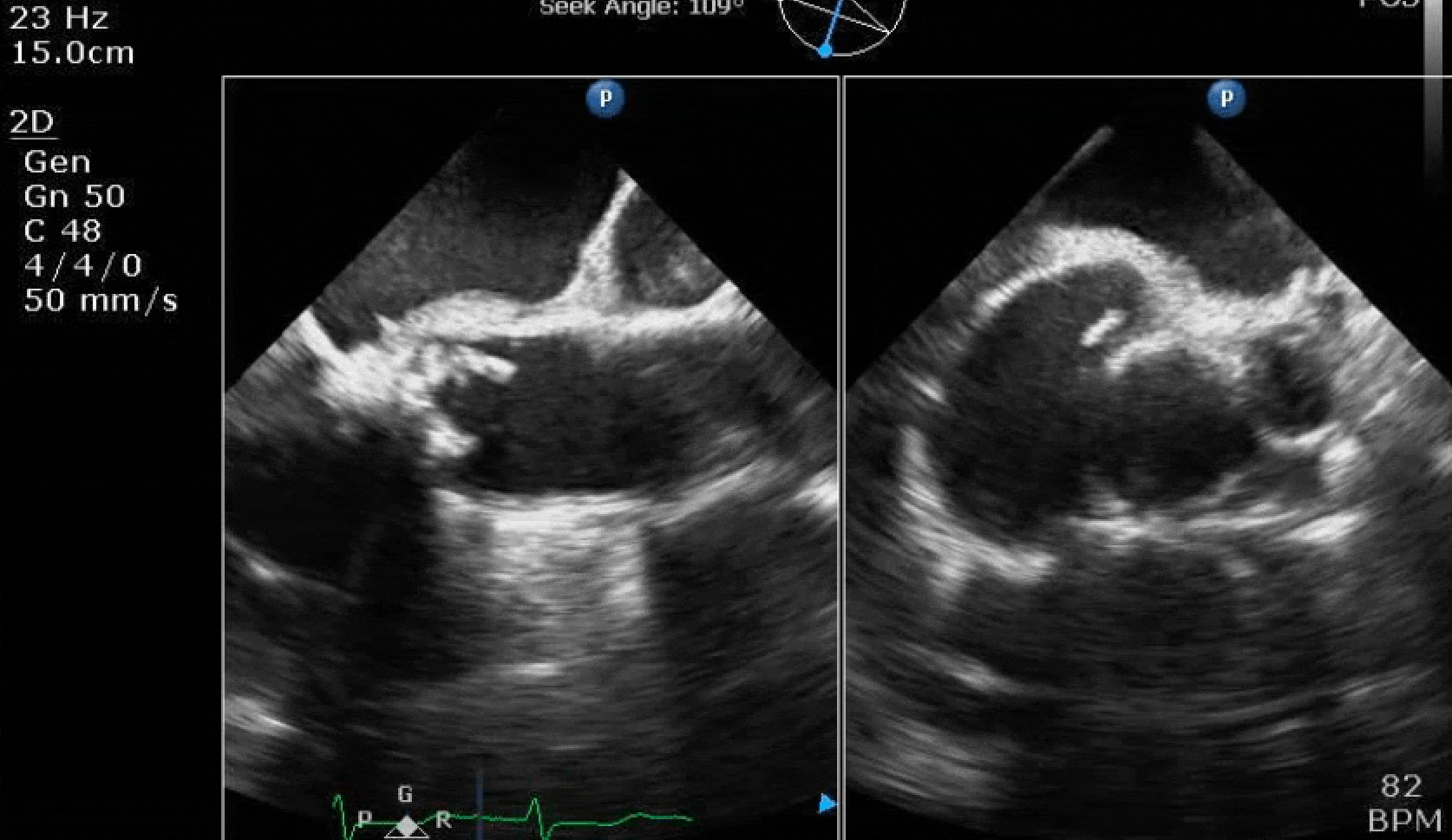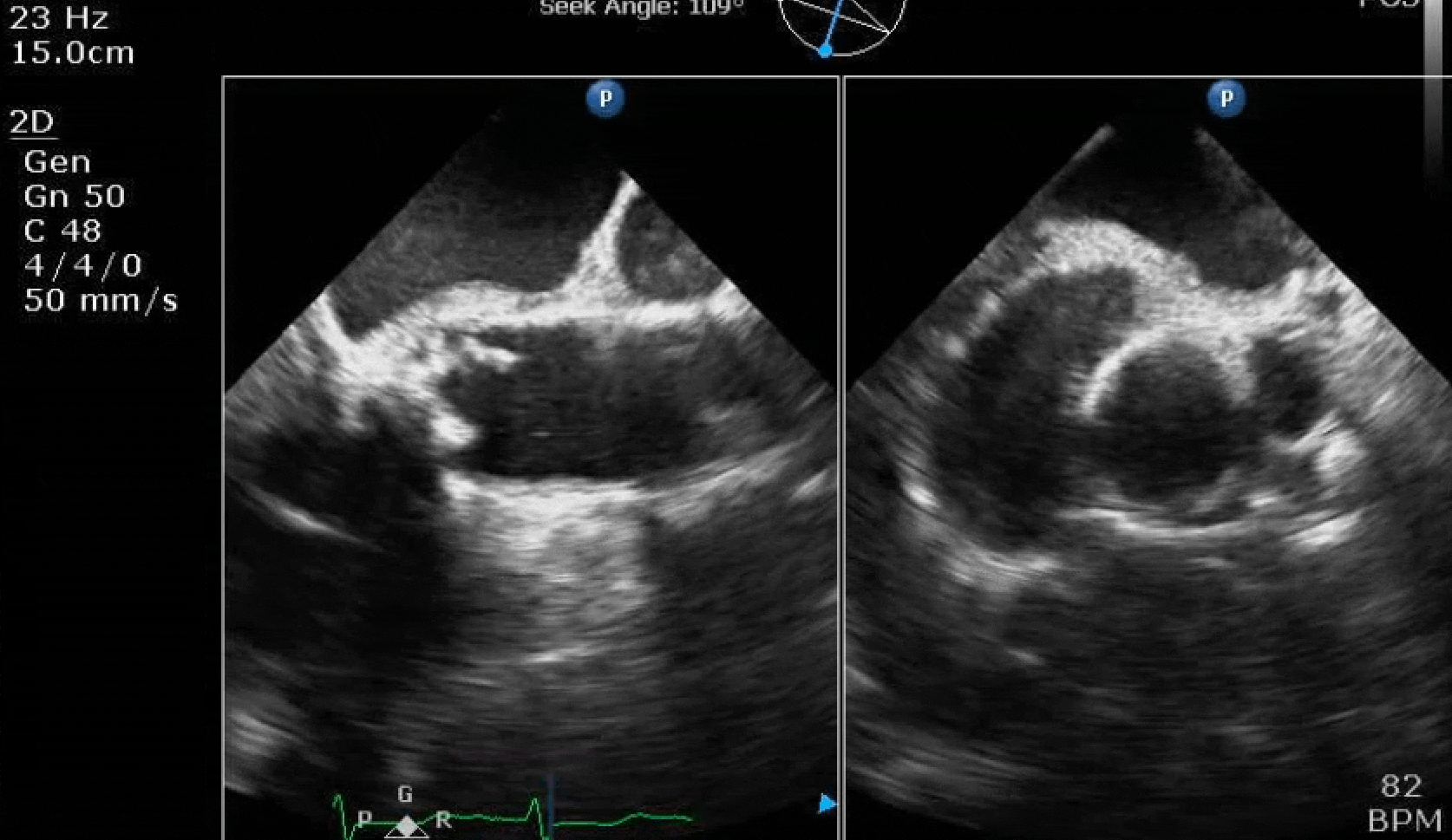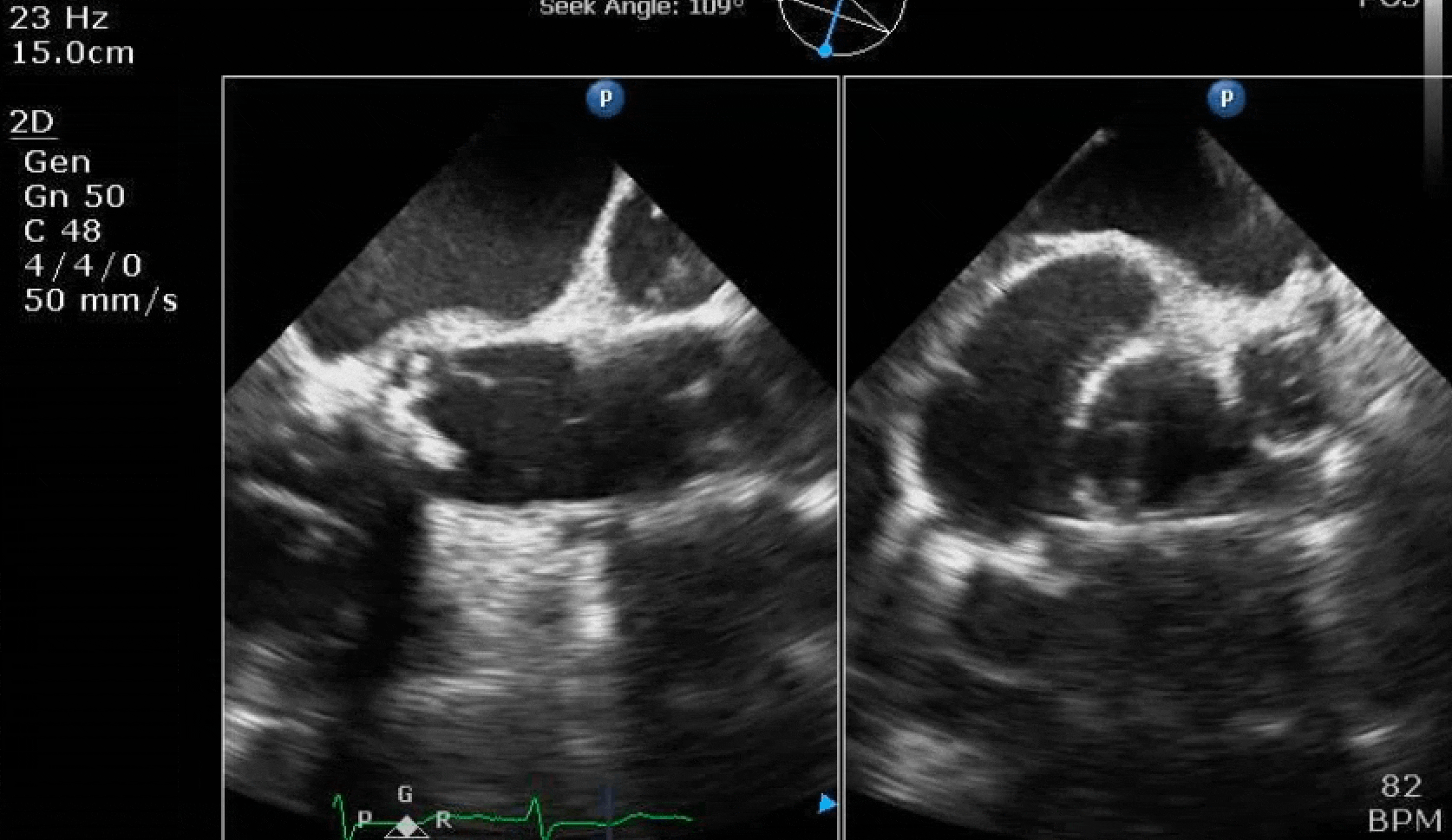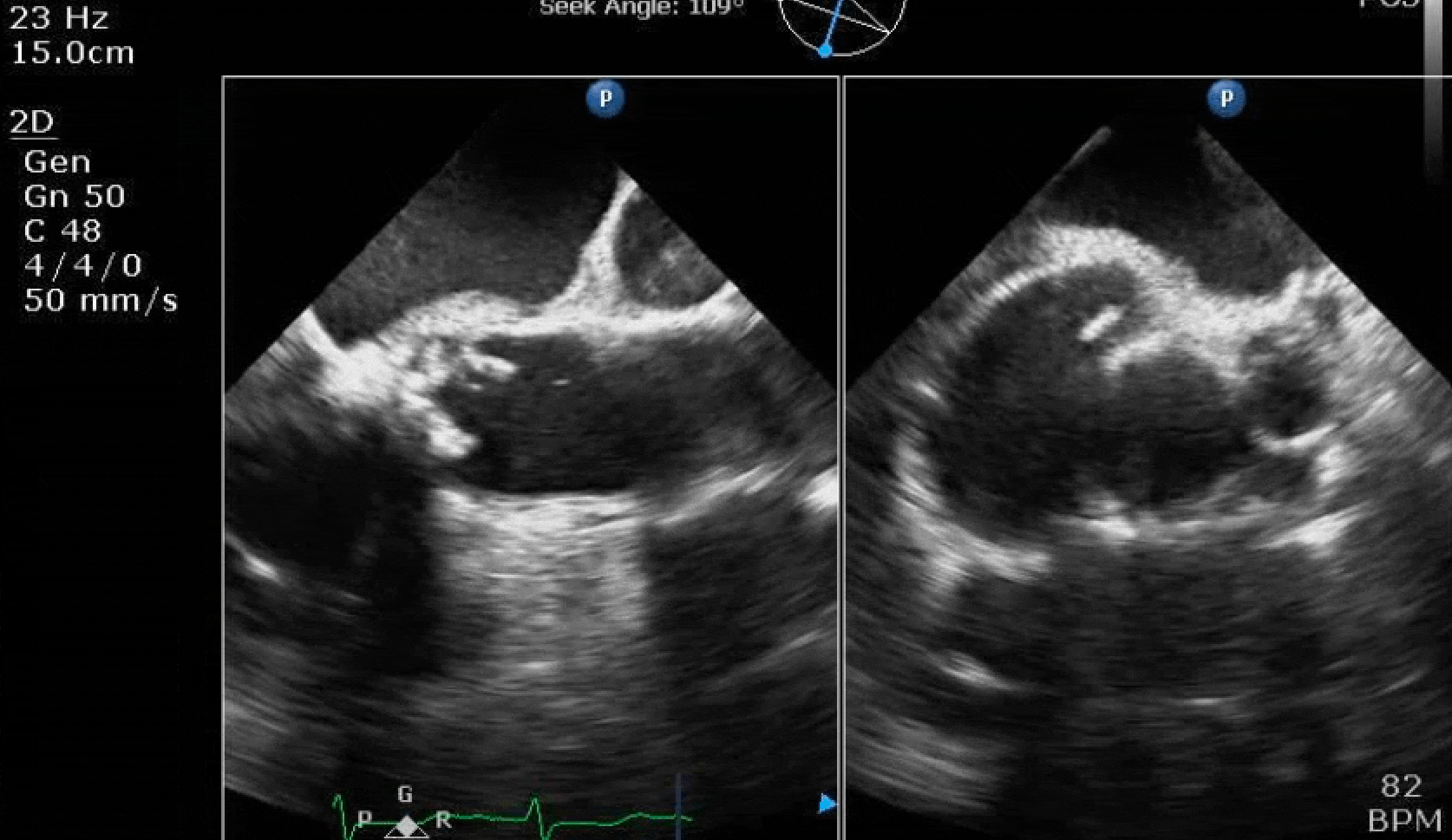
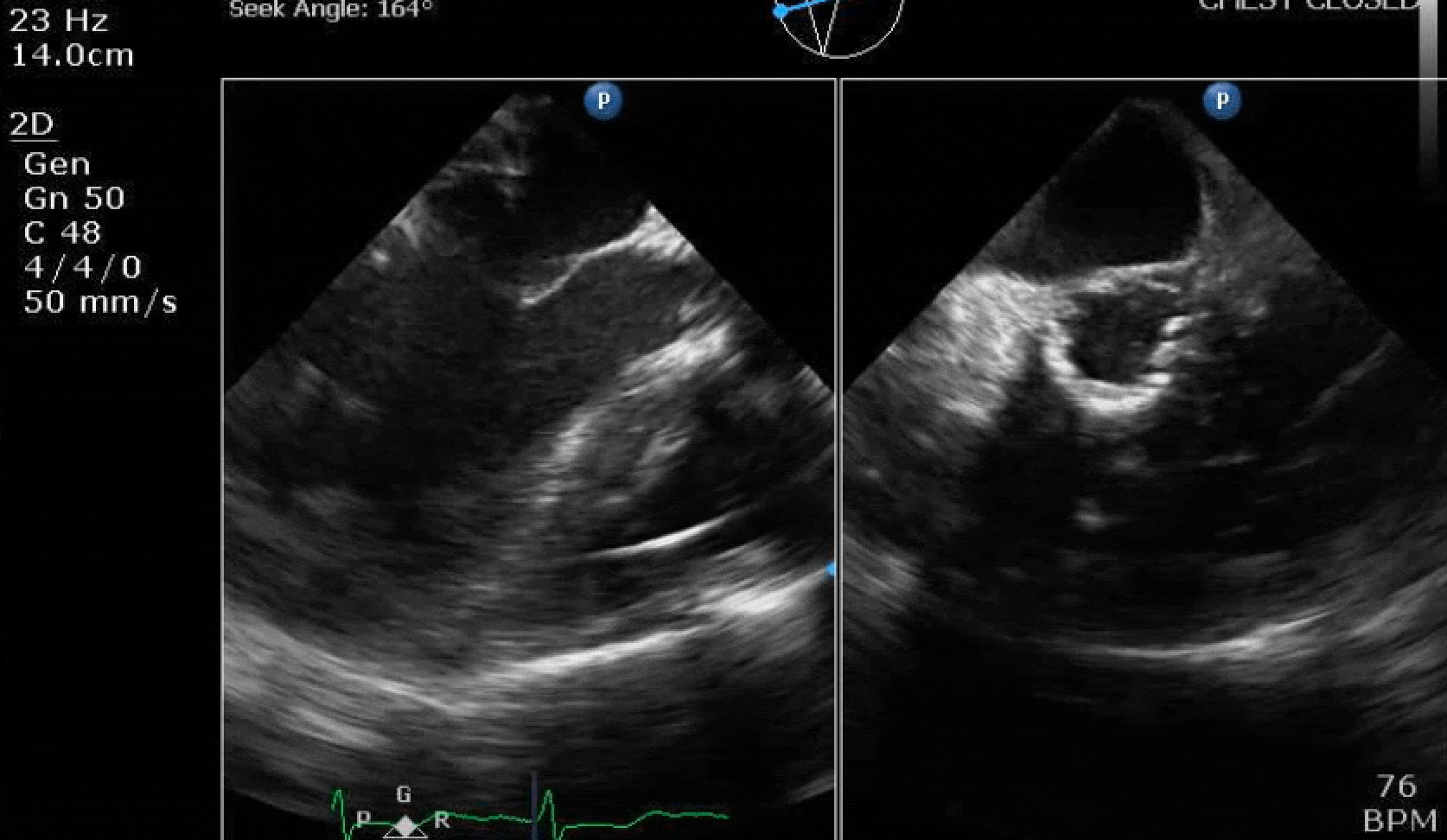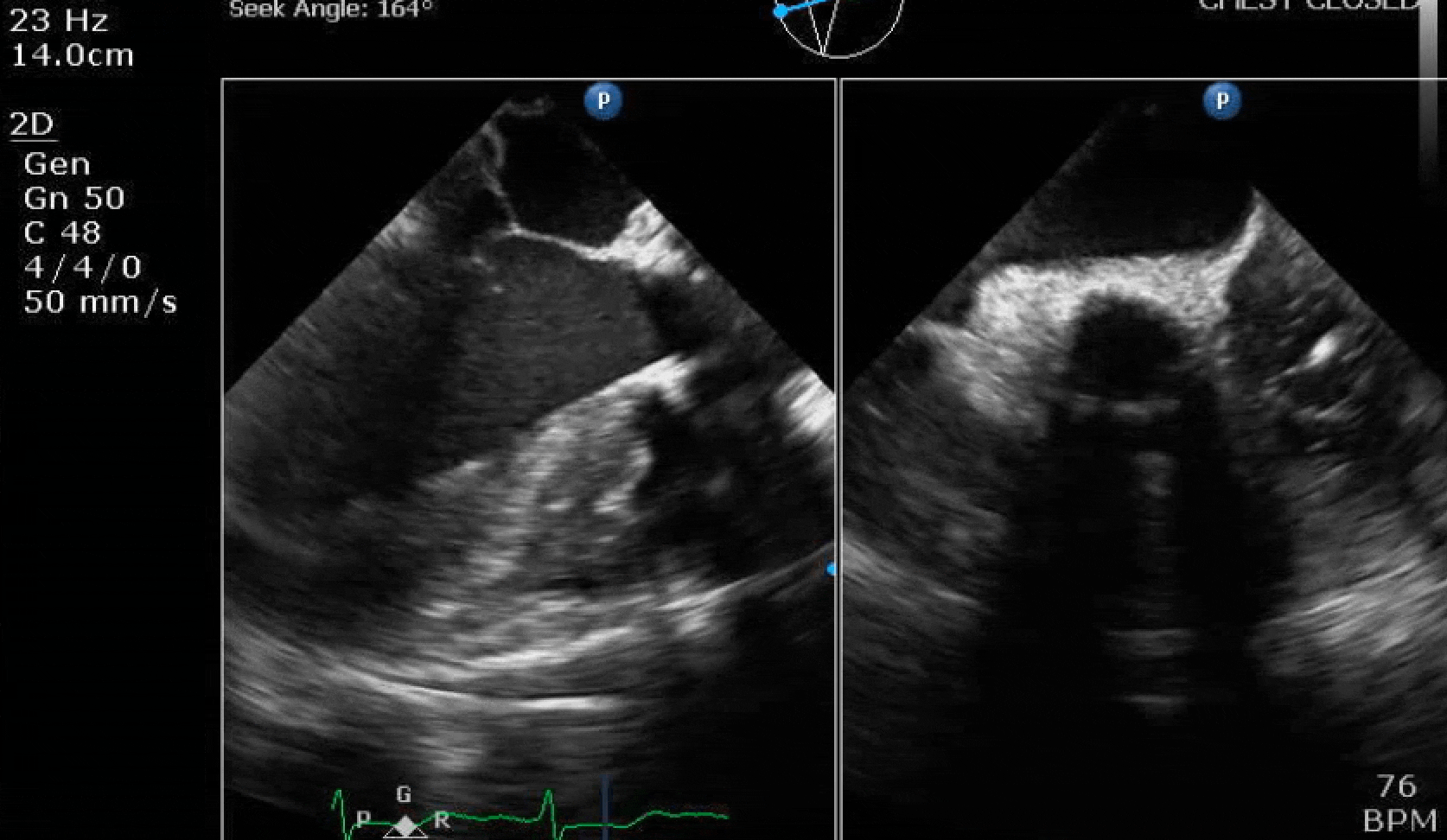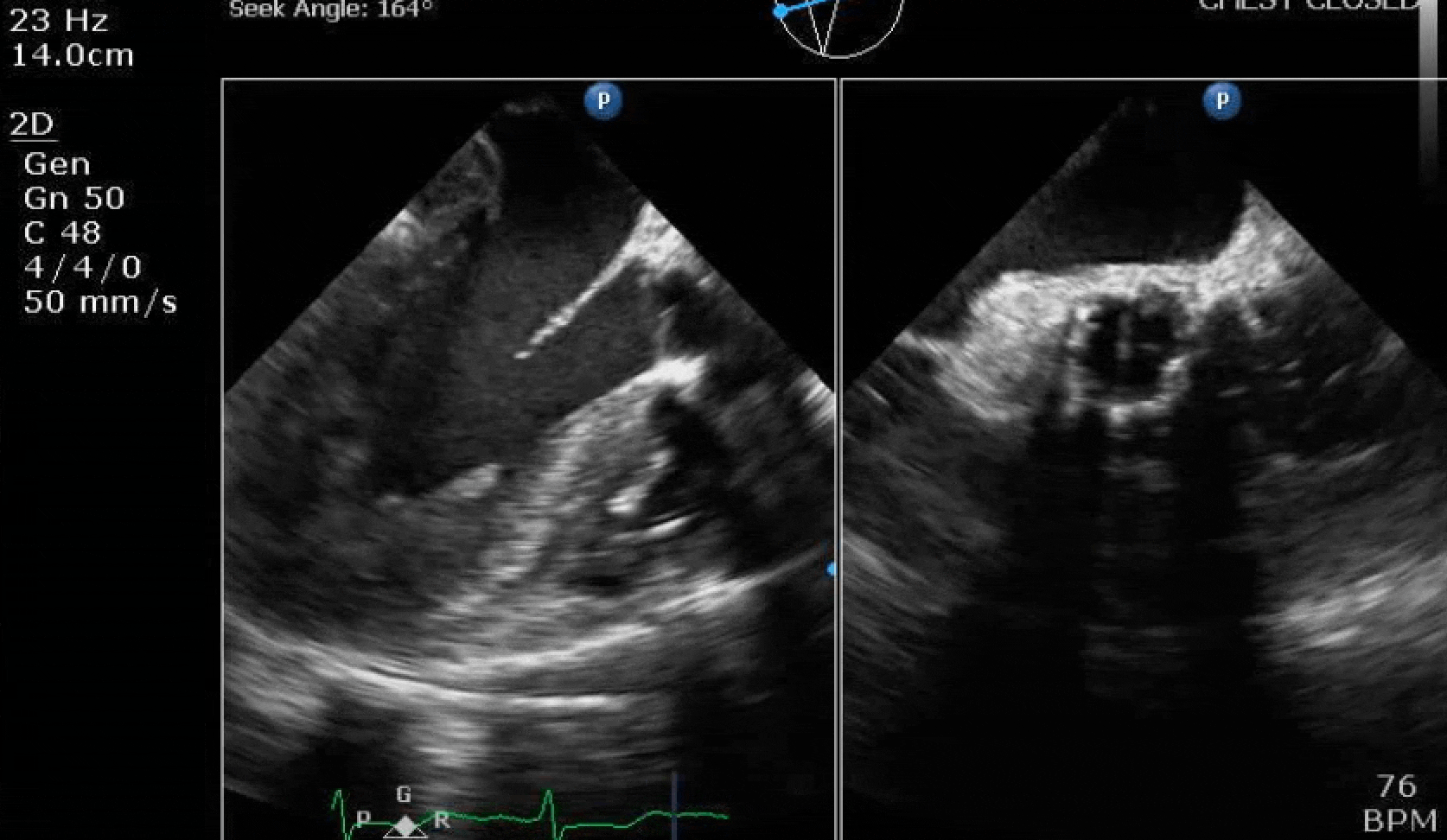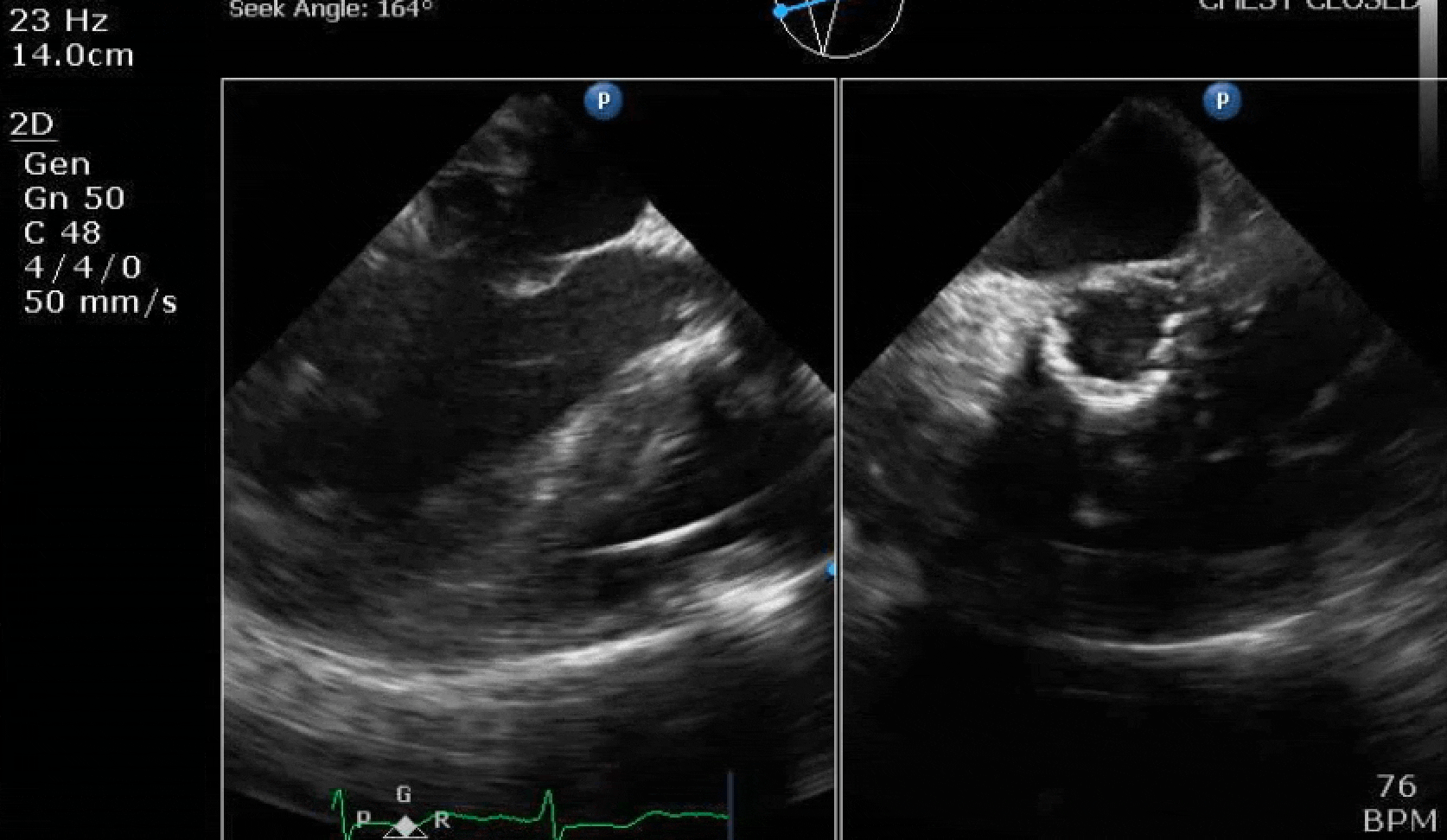
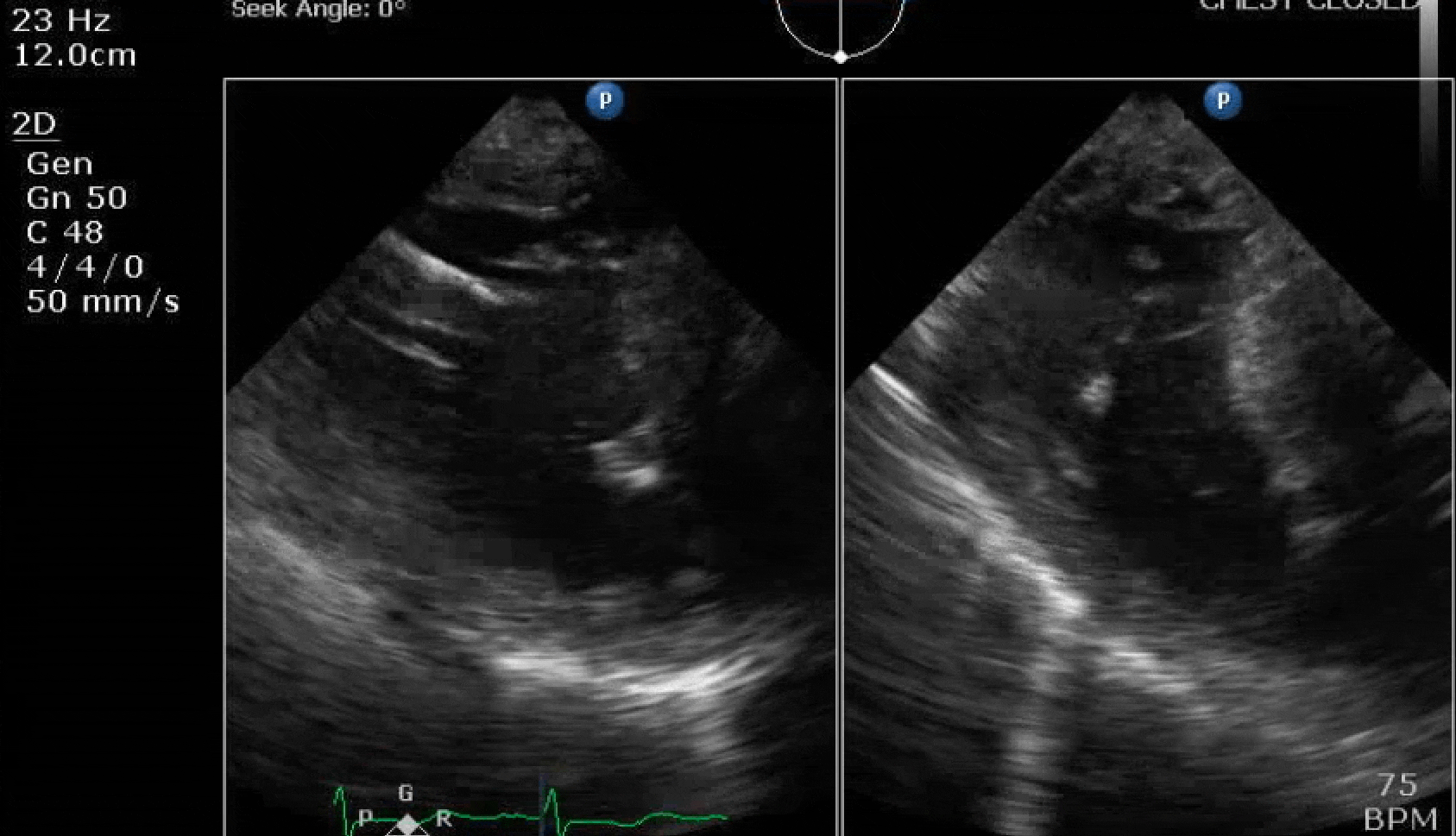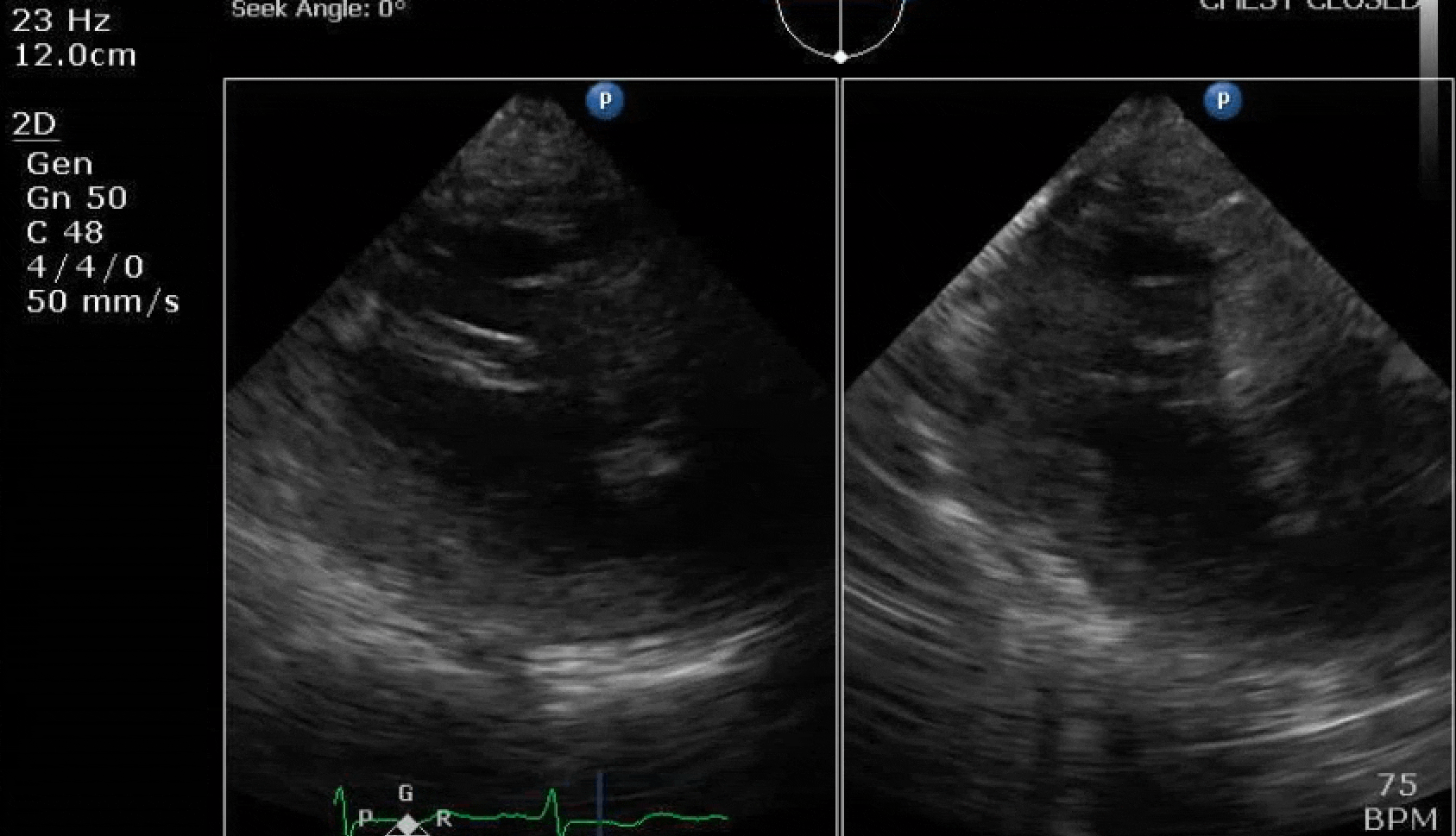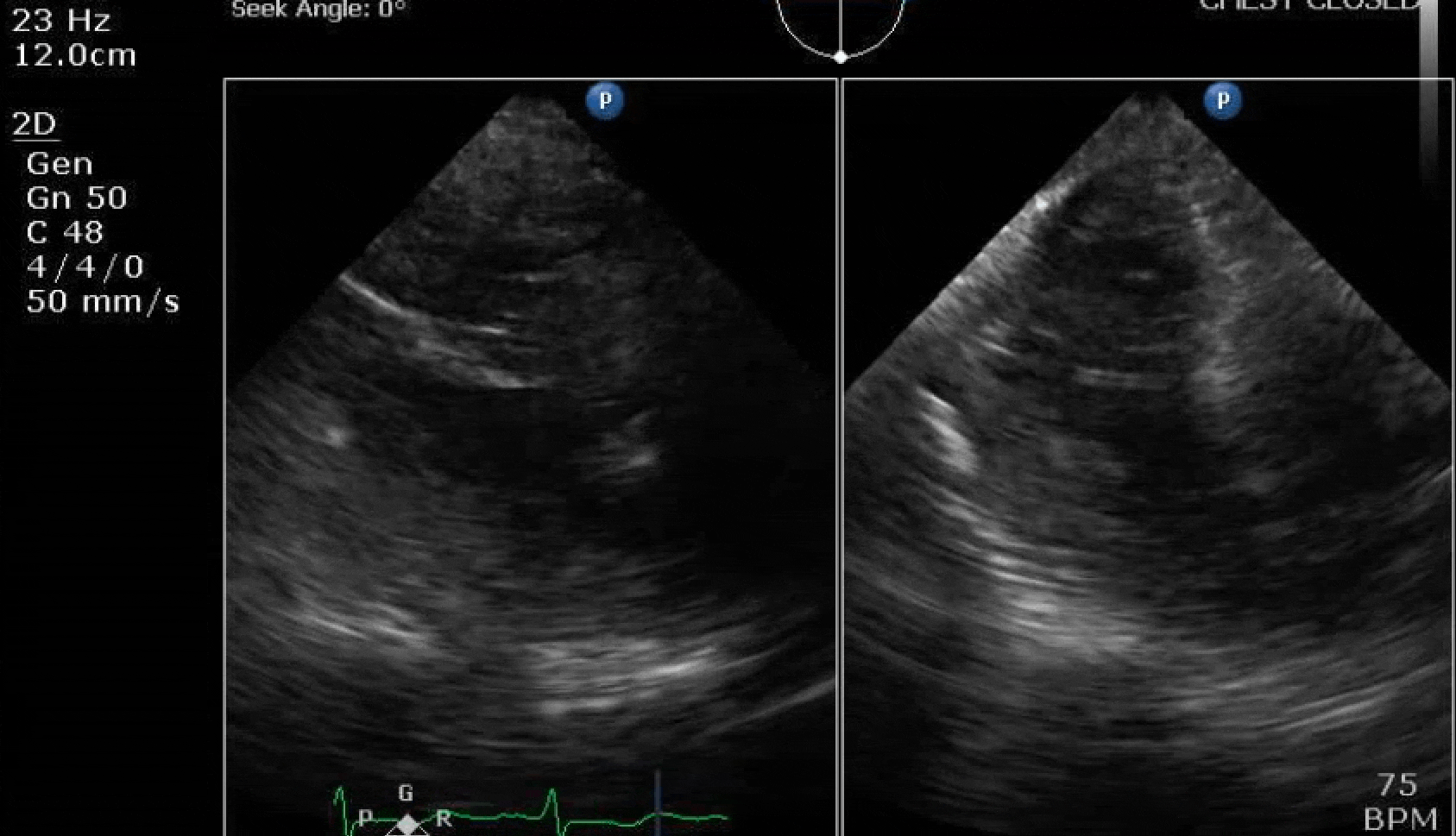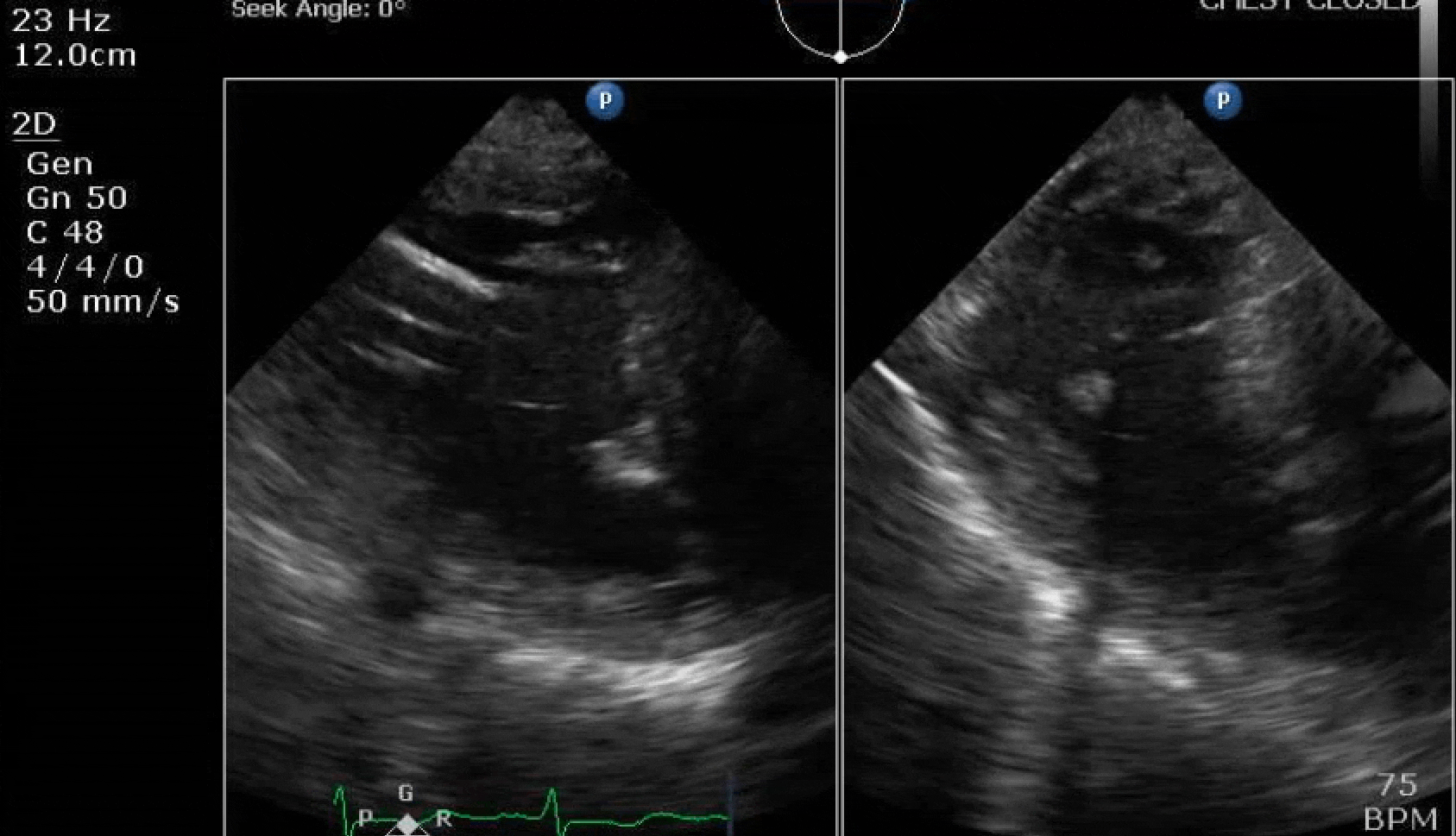
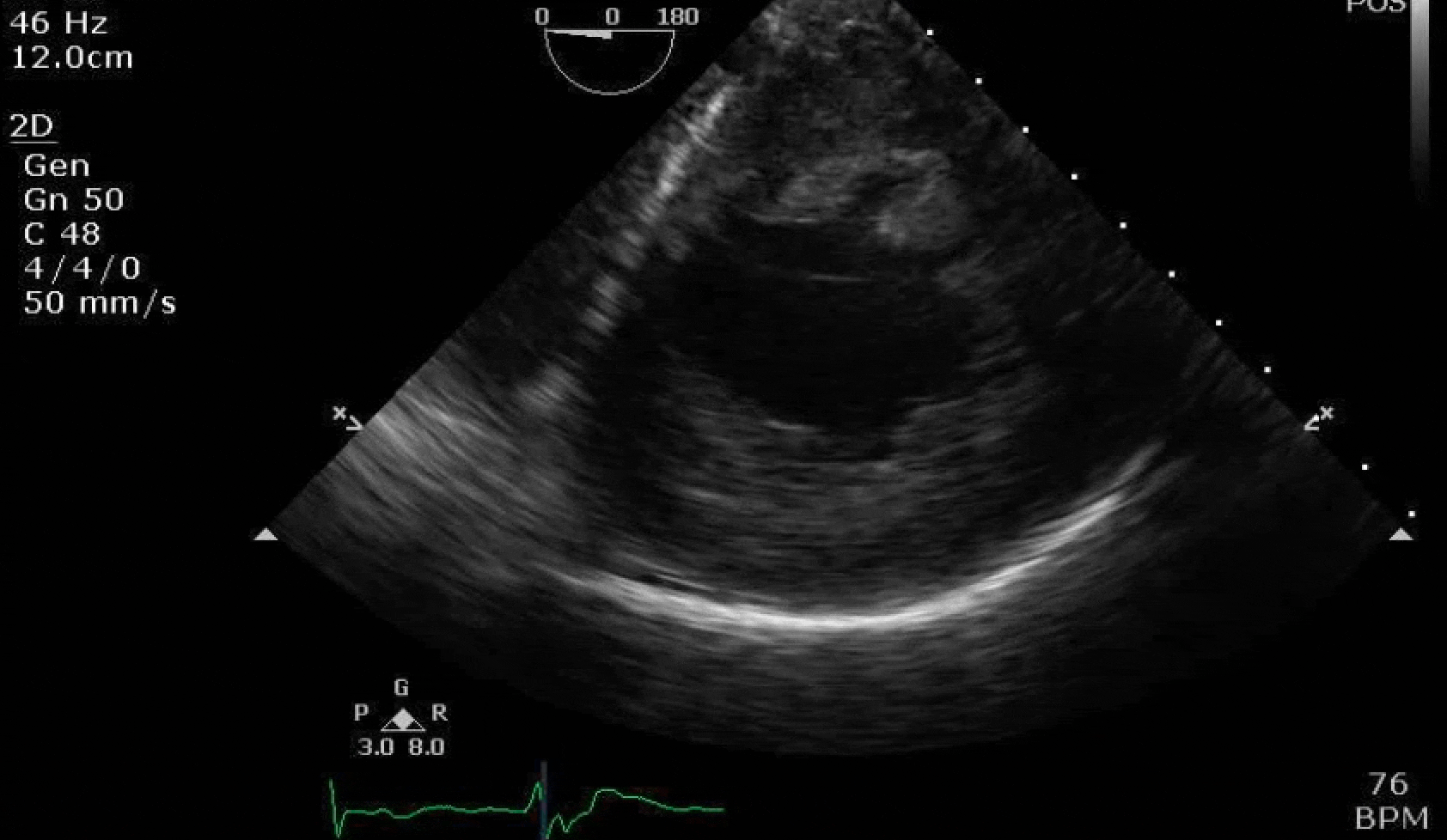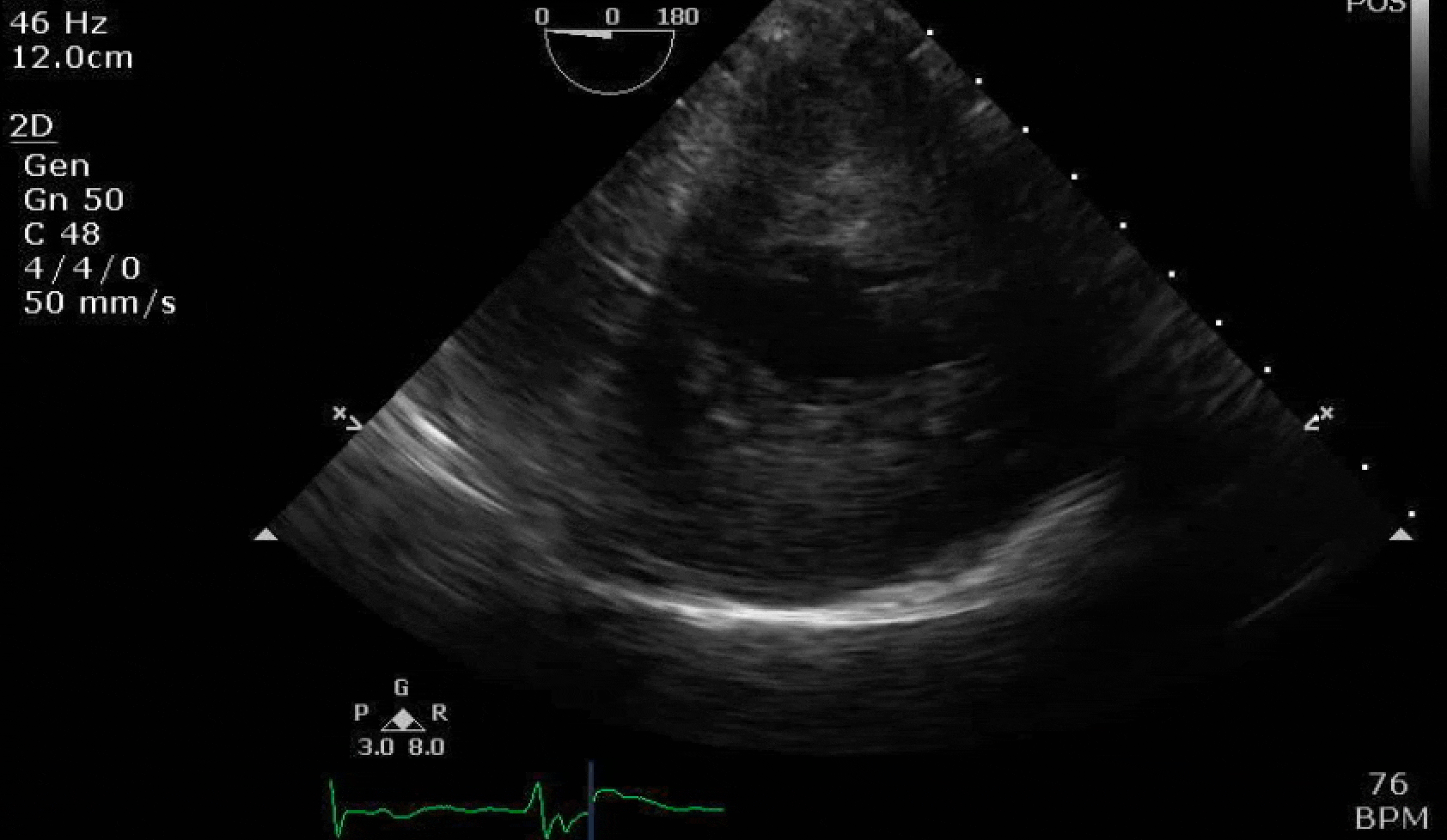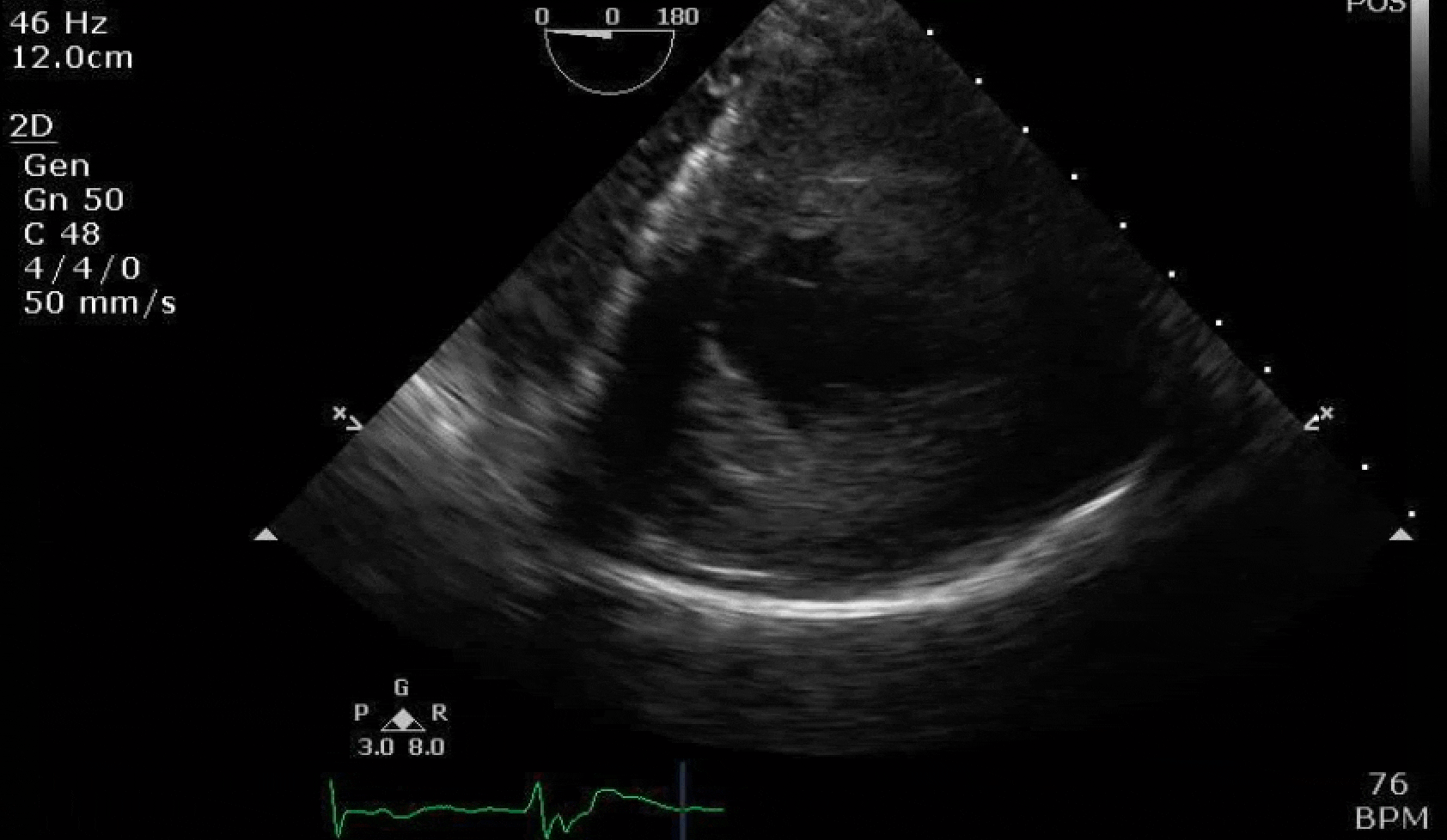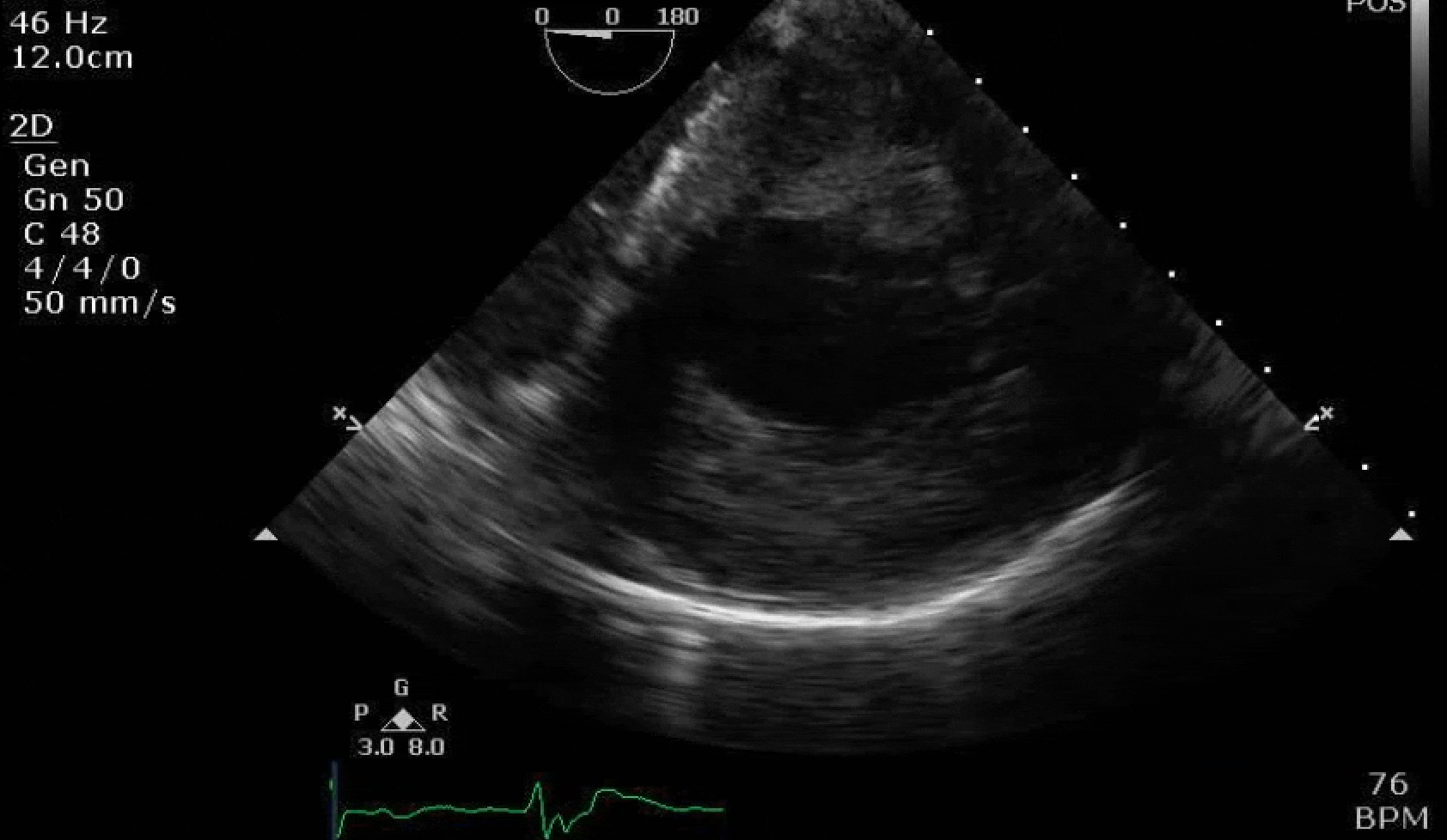
70 yo M, blue collar worker (active at baseline), no reported PMH, never seen a doctor. Walks into the ED with 3 day hx of dyspnea and 2-3 weeks of new lower extremity edema. Also has a URI. HR 101, BP 97/55, satting 96 on RA. Super high BNP, negative trop. Admitted for new HF. CT chest shows incidental 5.0 cm ascending aorta and also small pleural effusions.
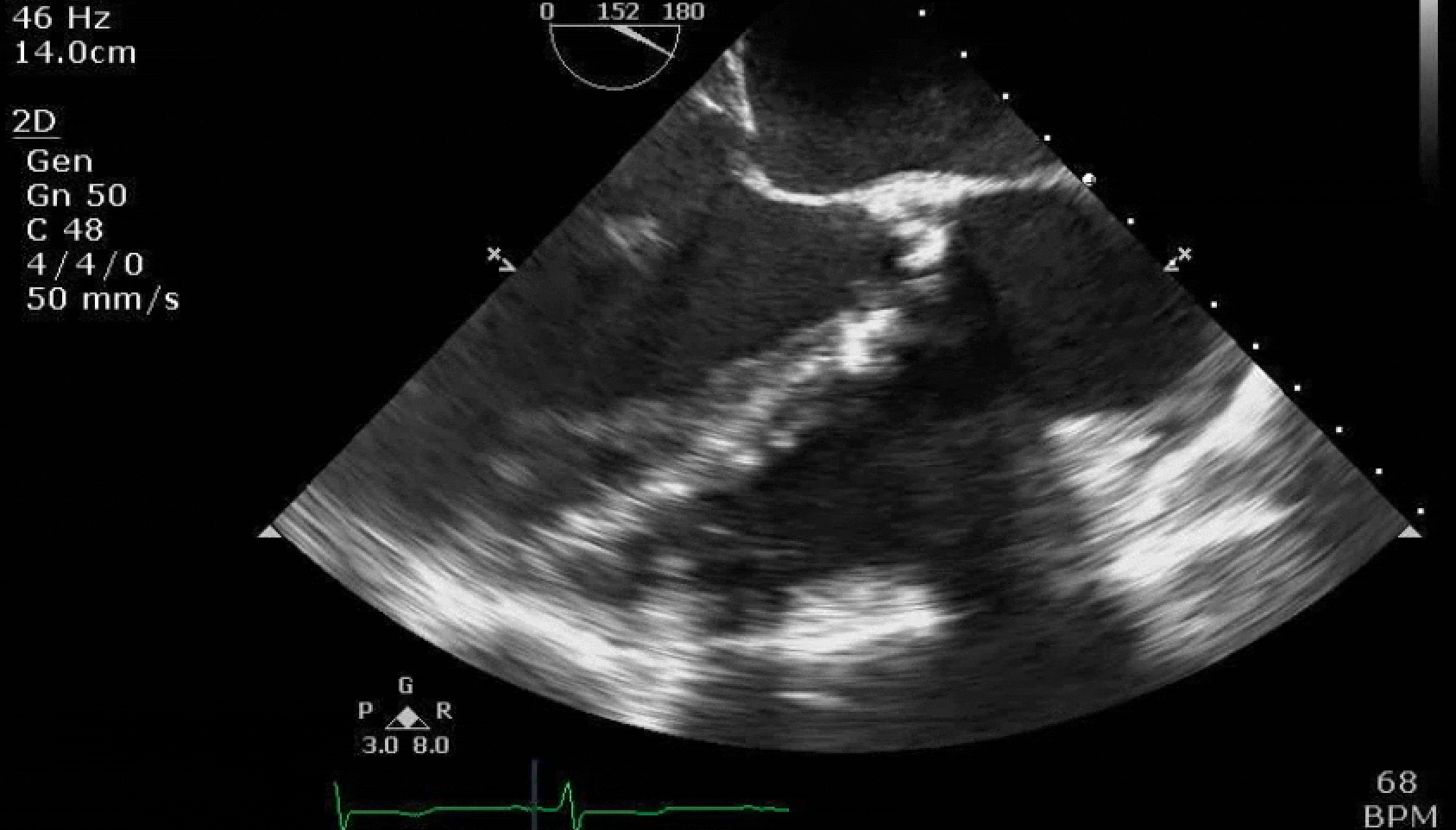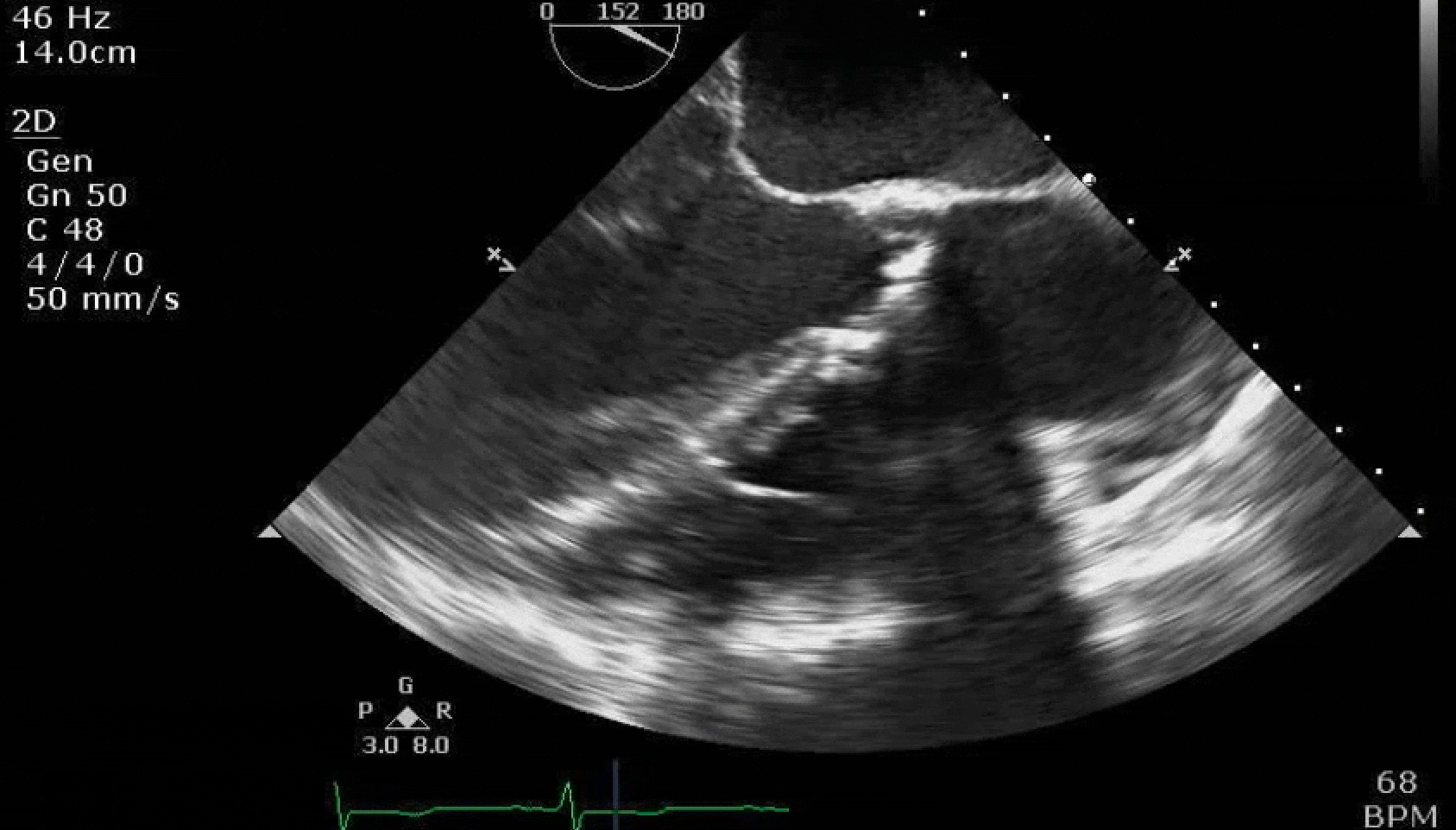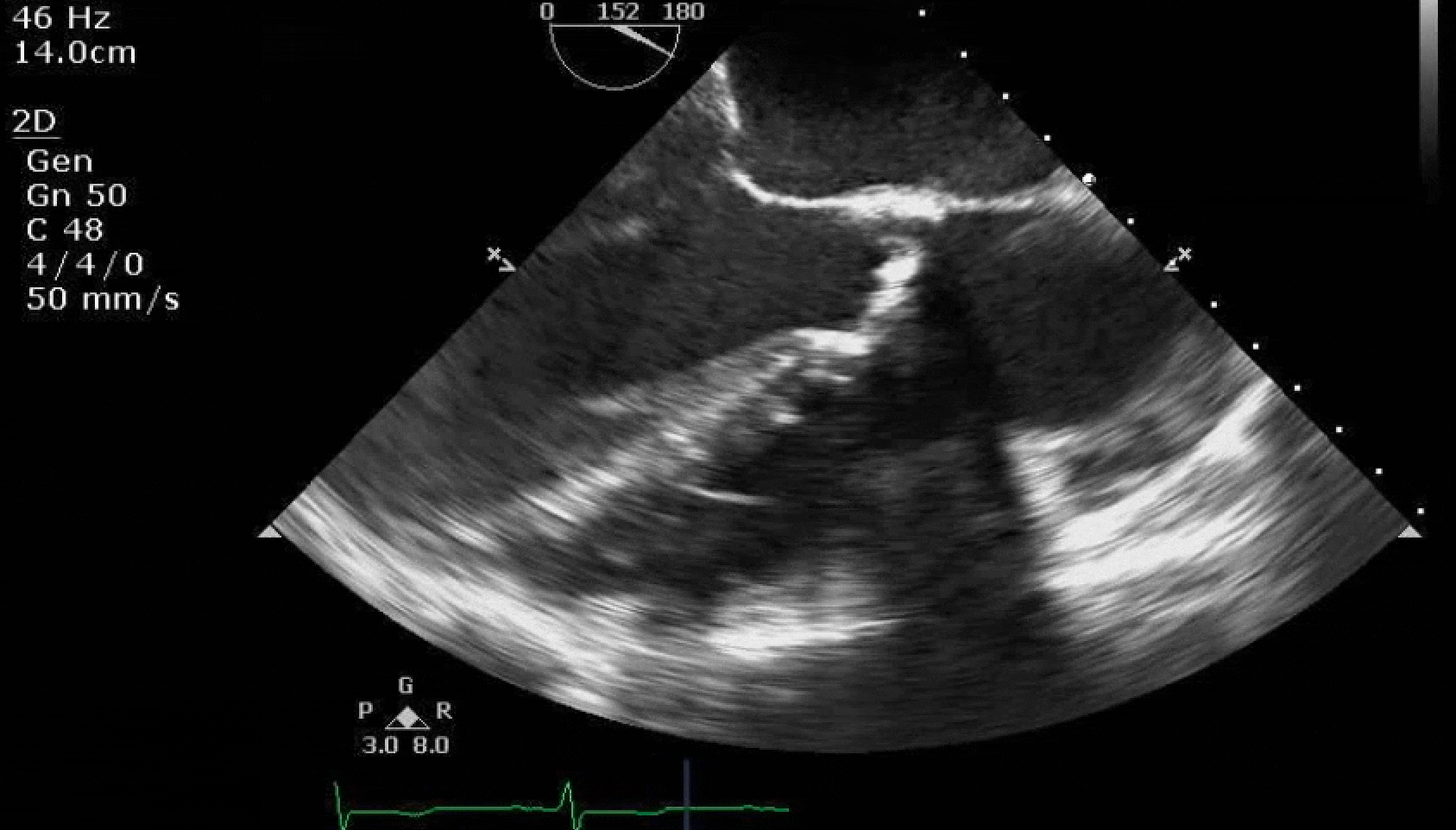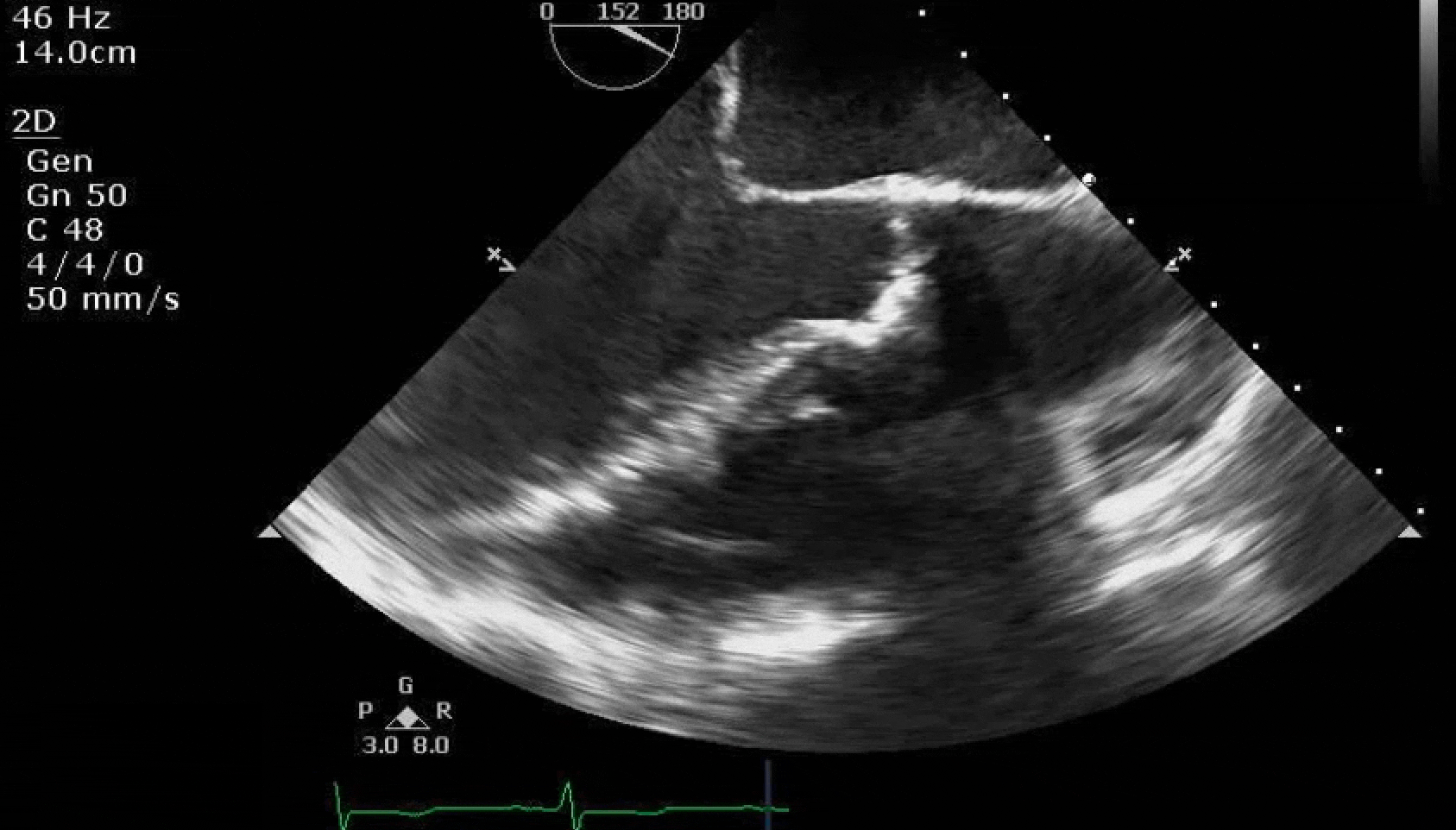
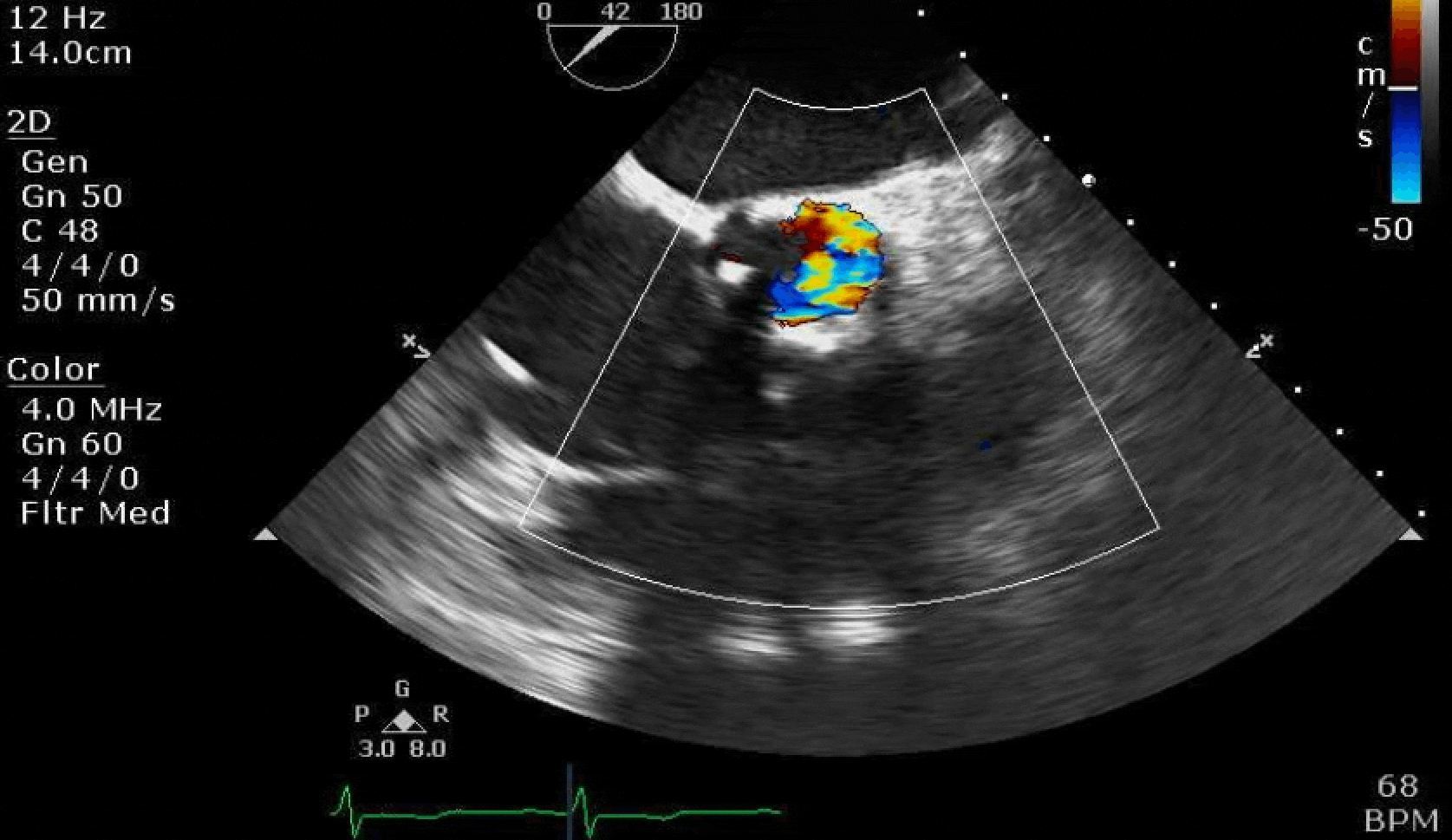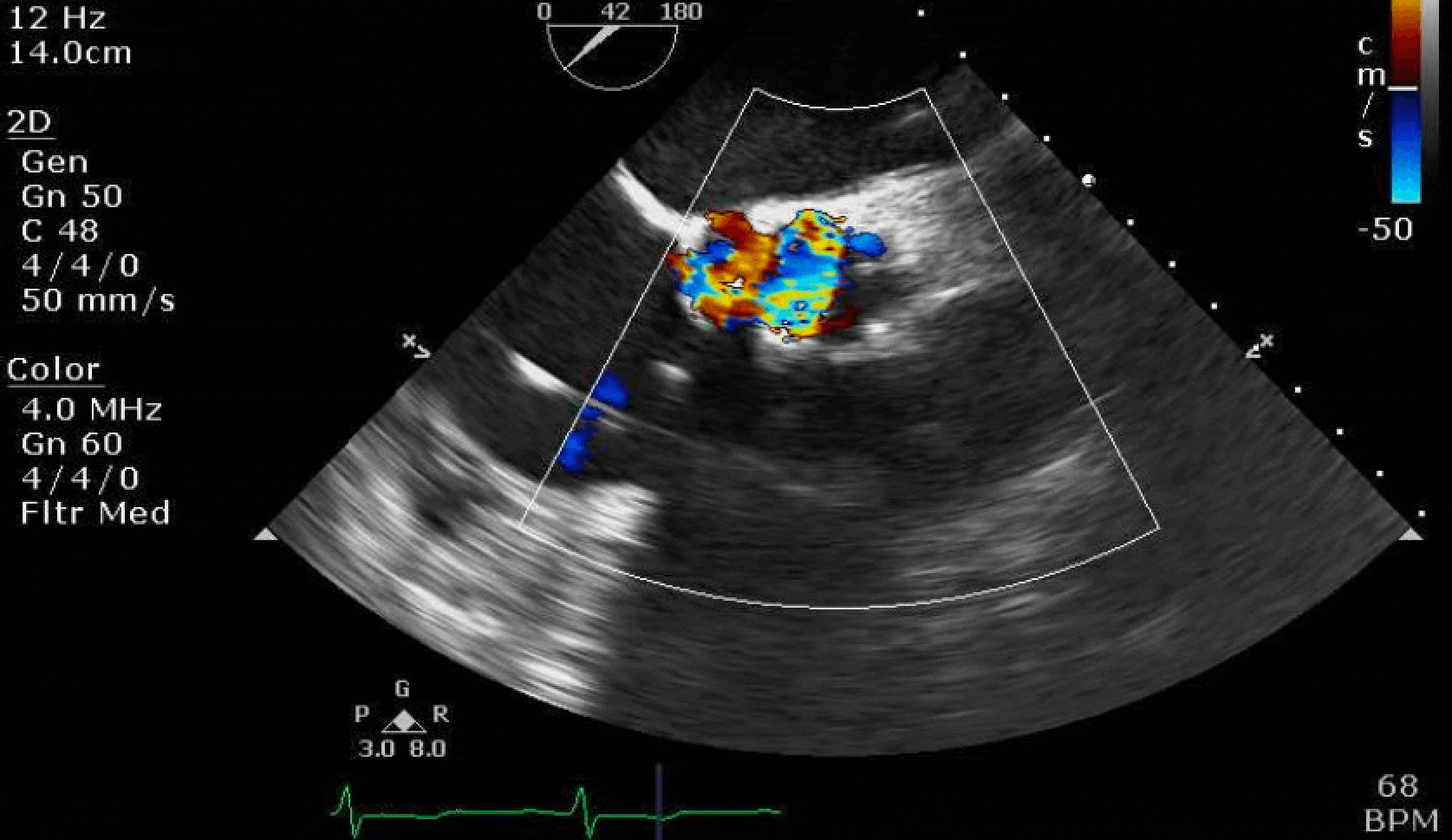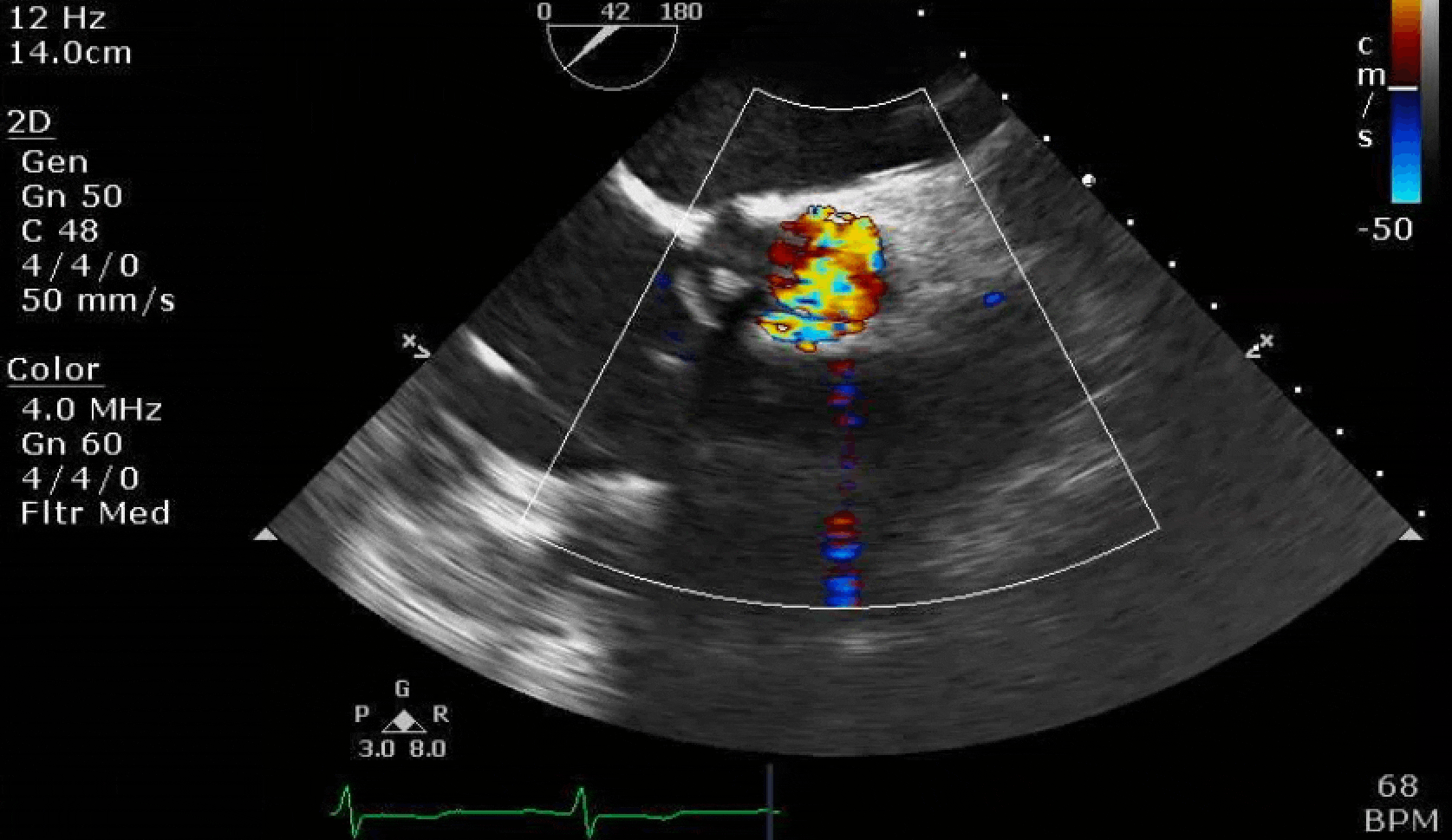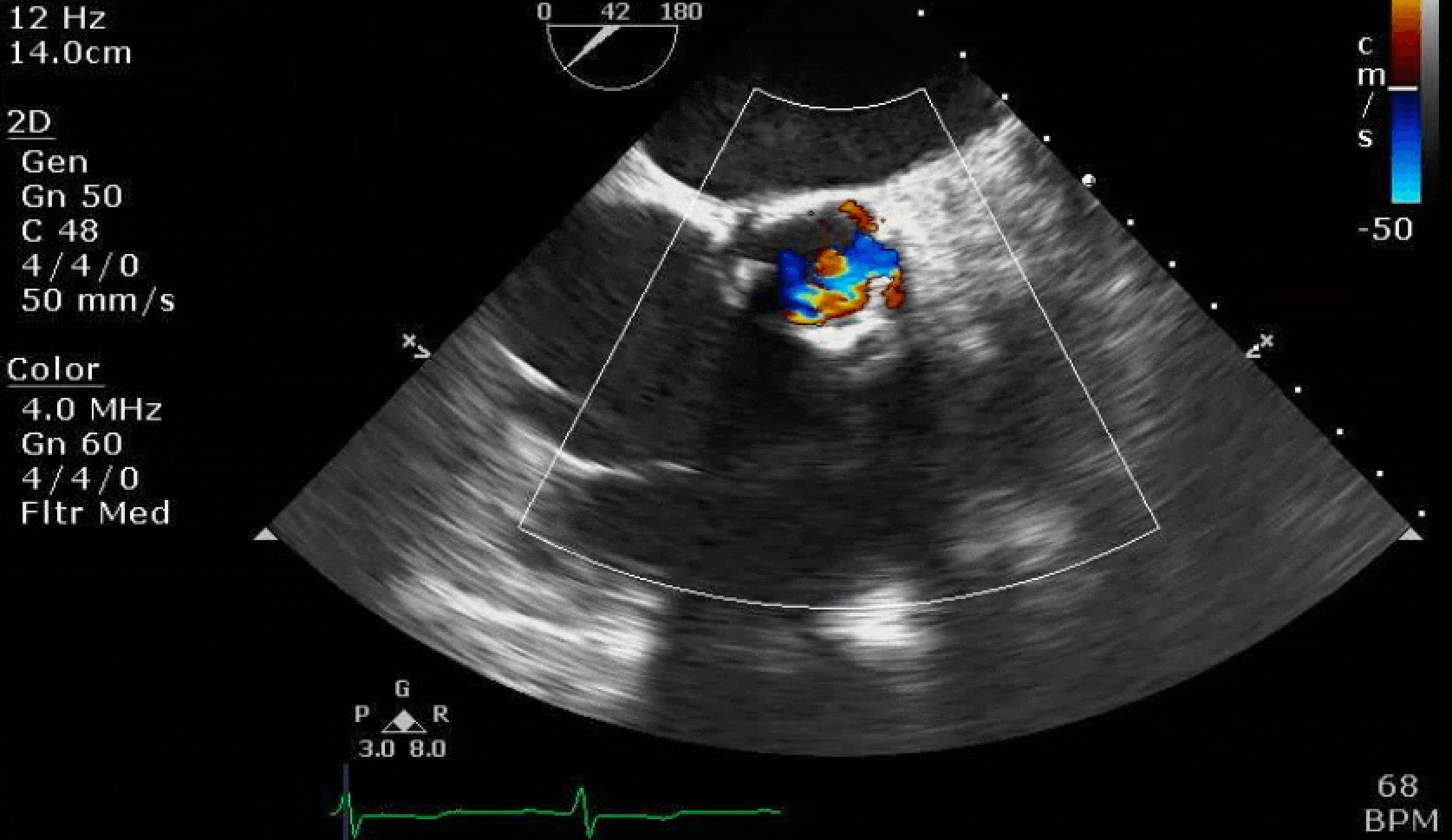
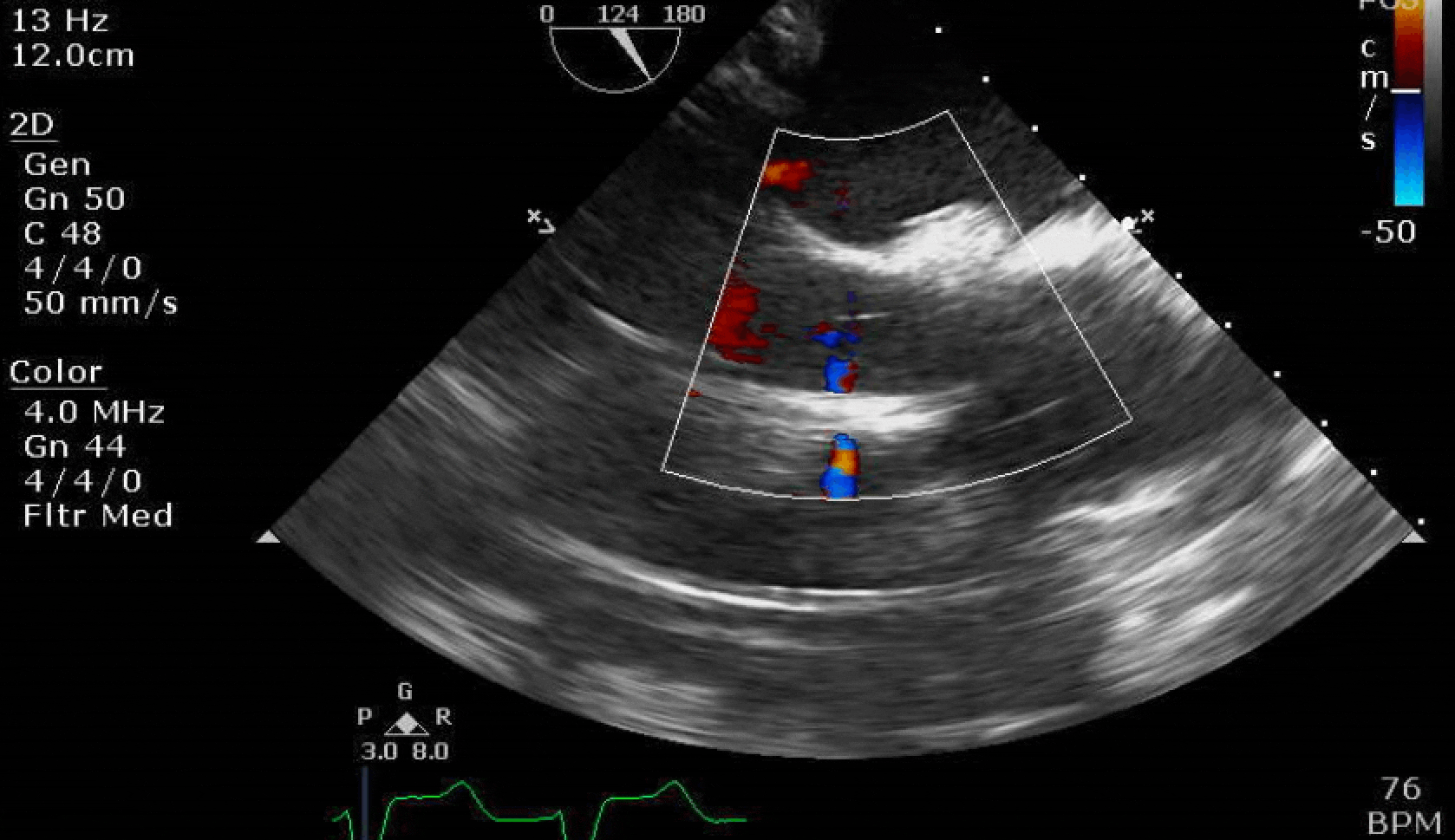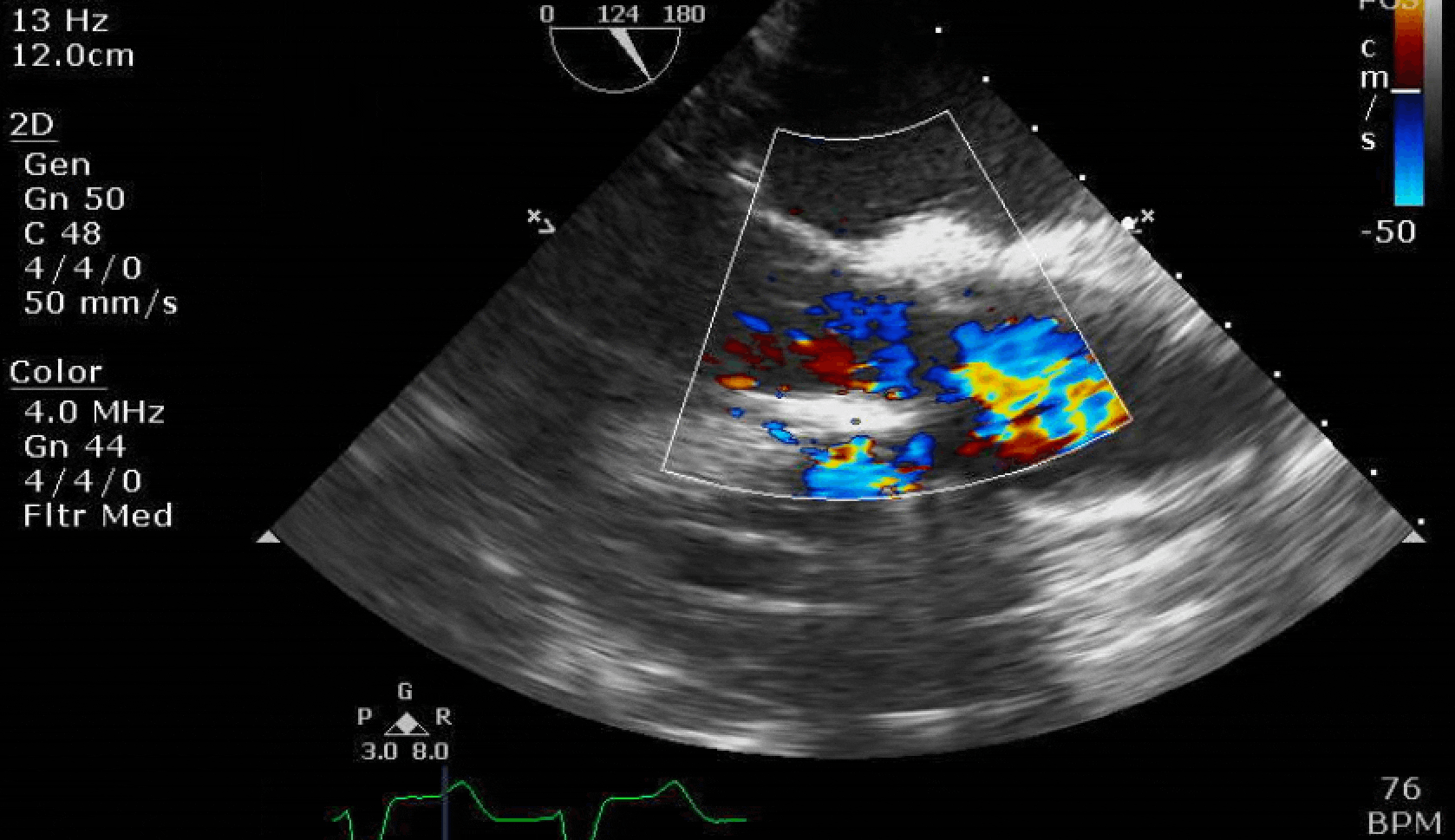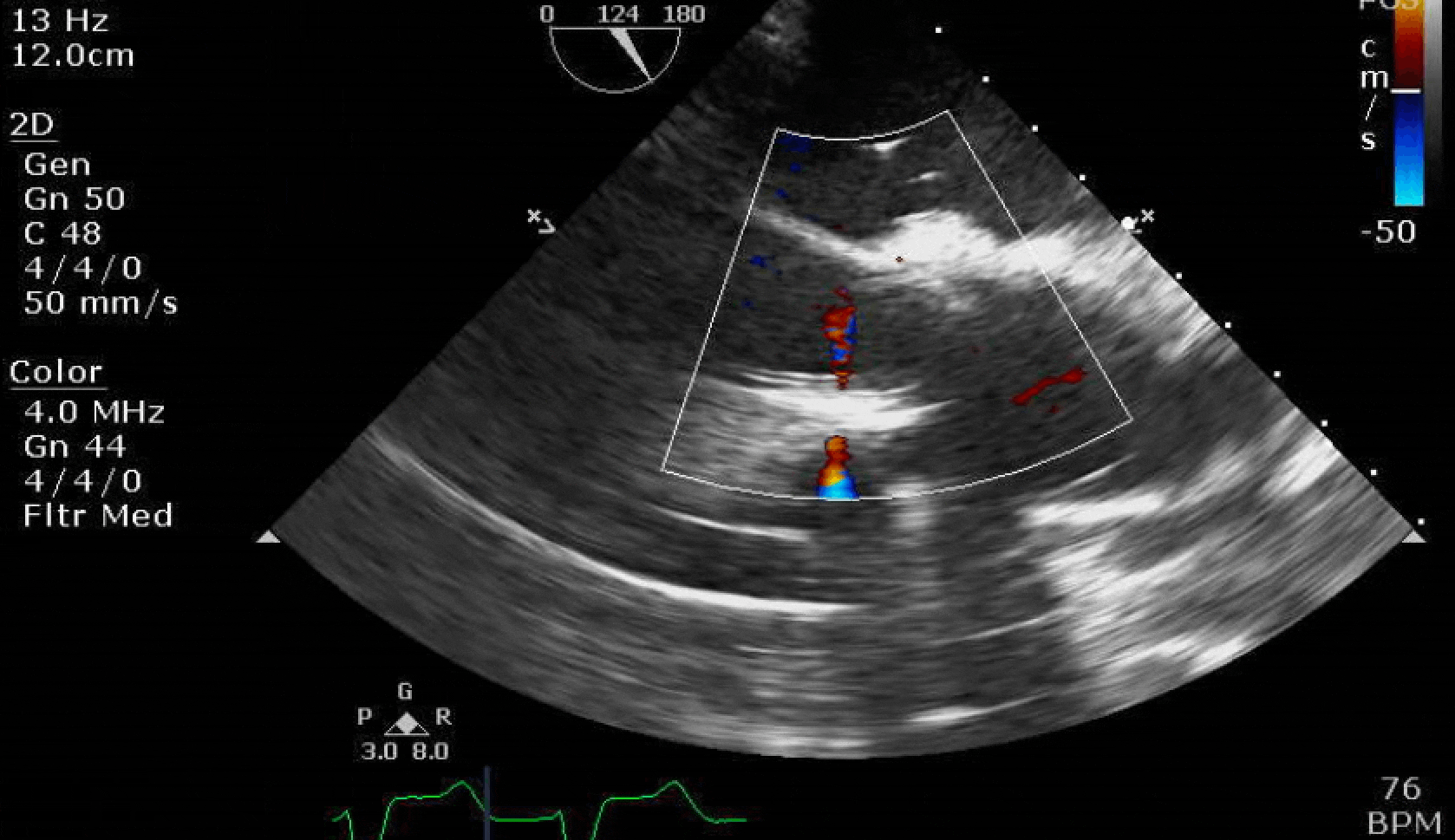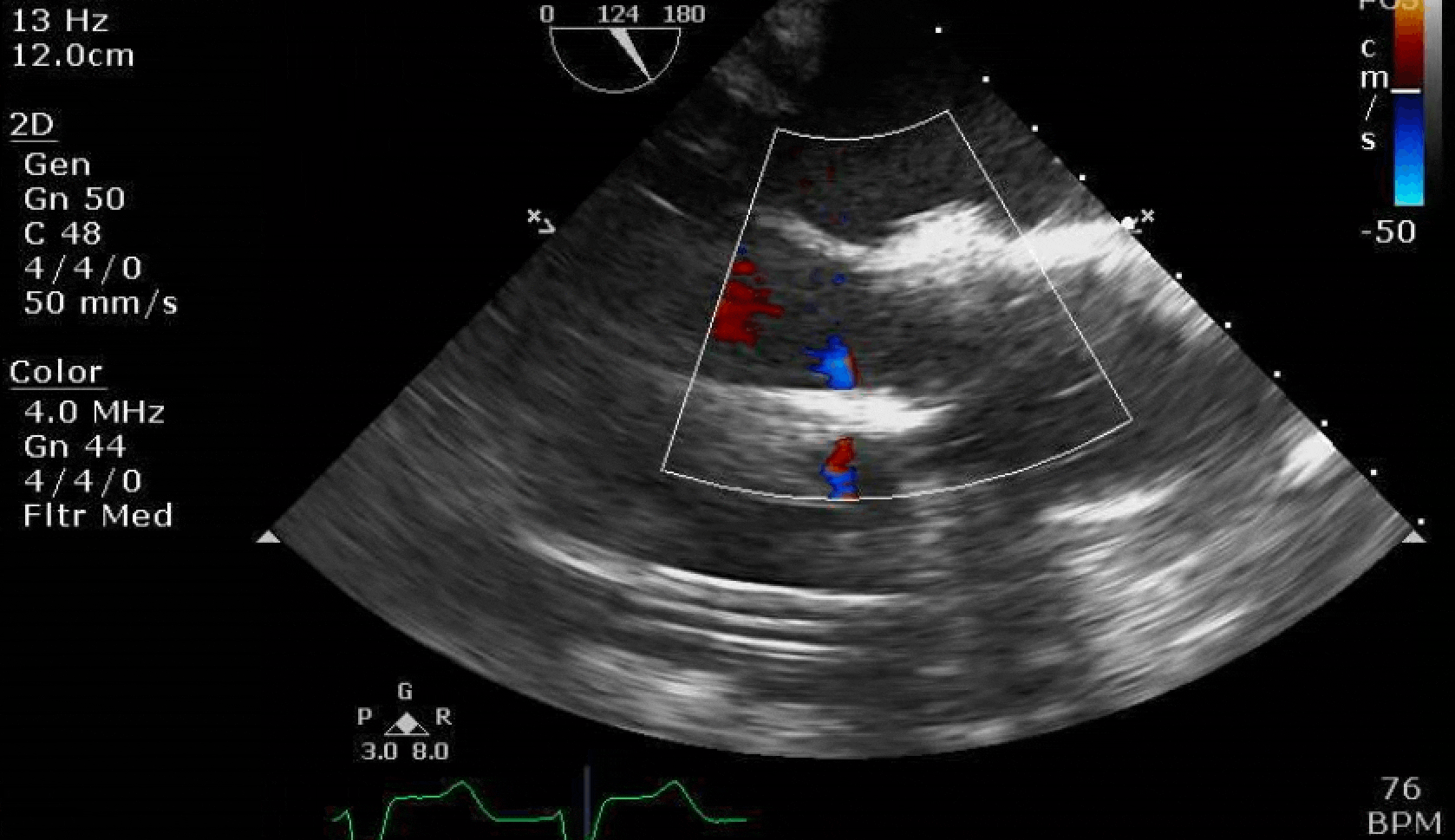
Here are some TTE instead of TEE images for a change.
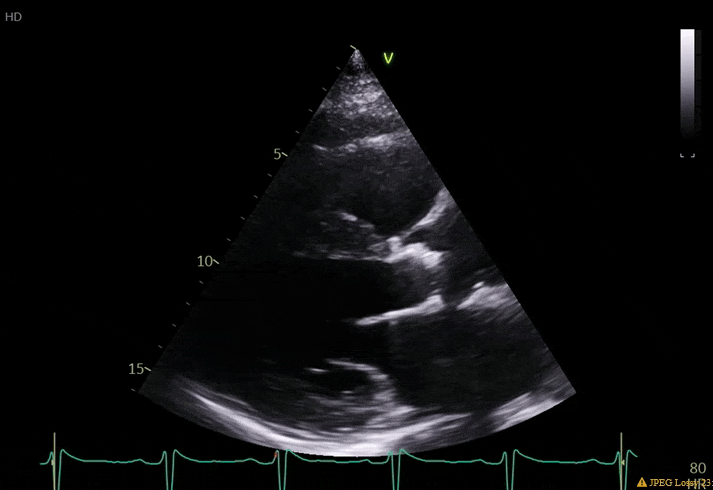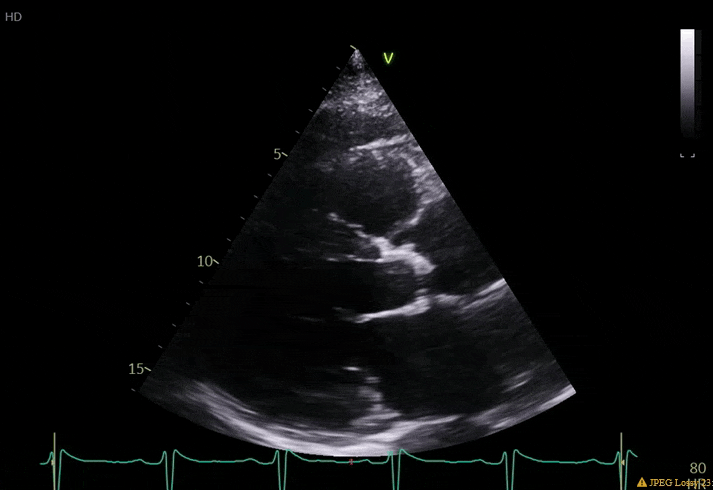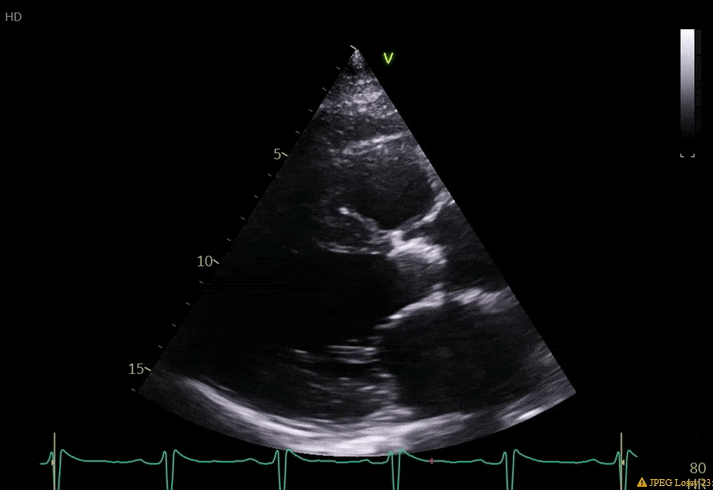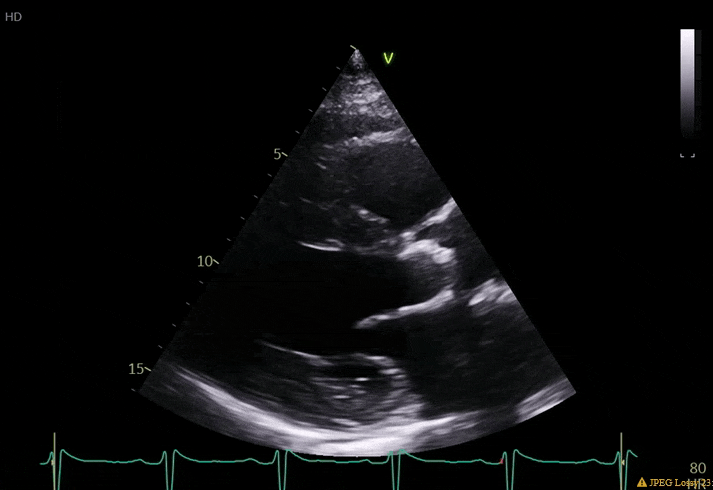
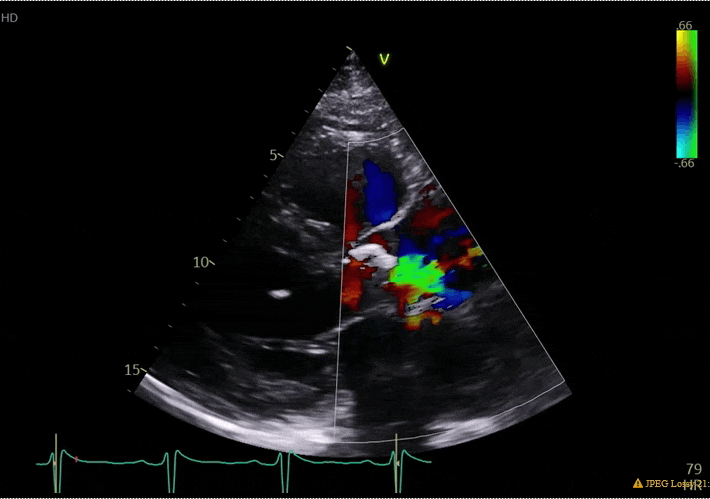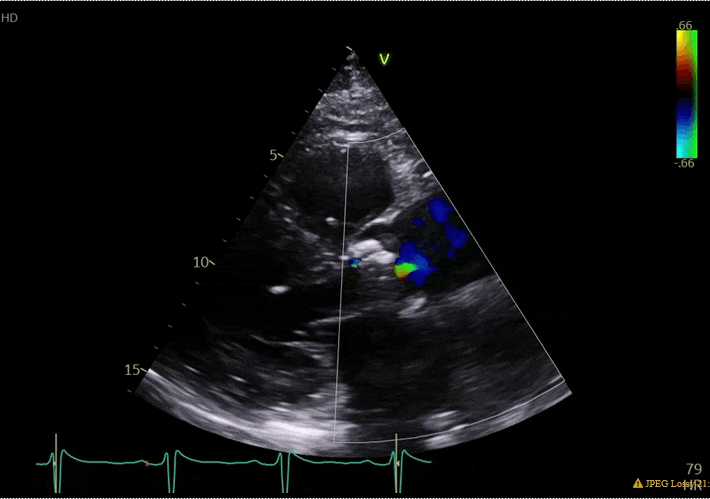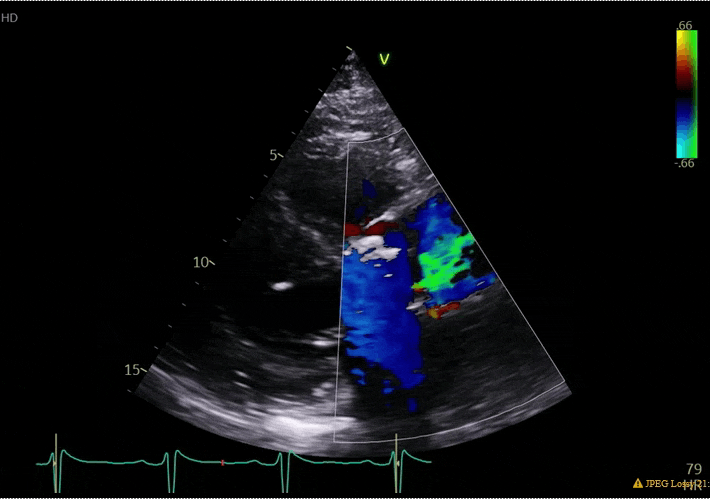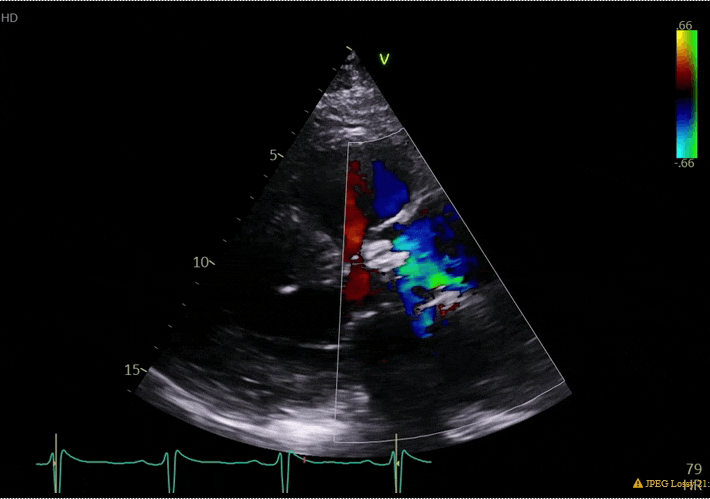
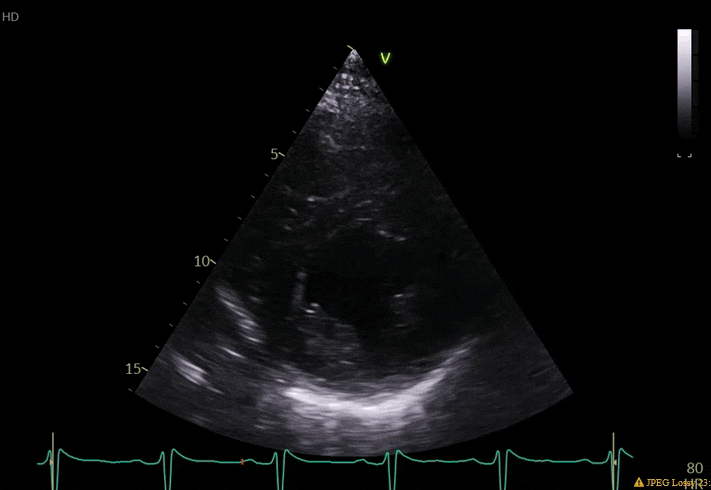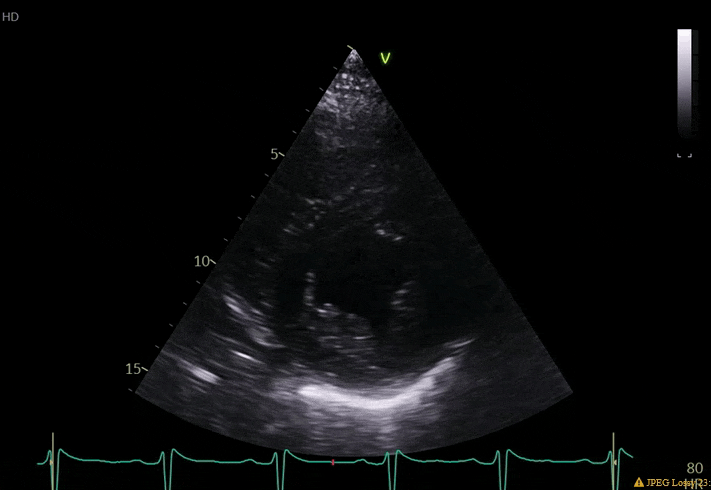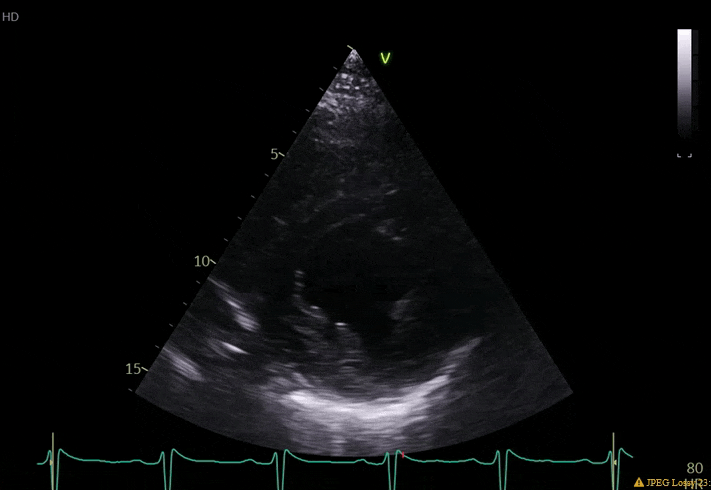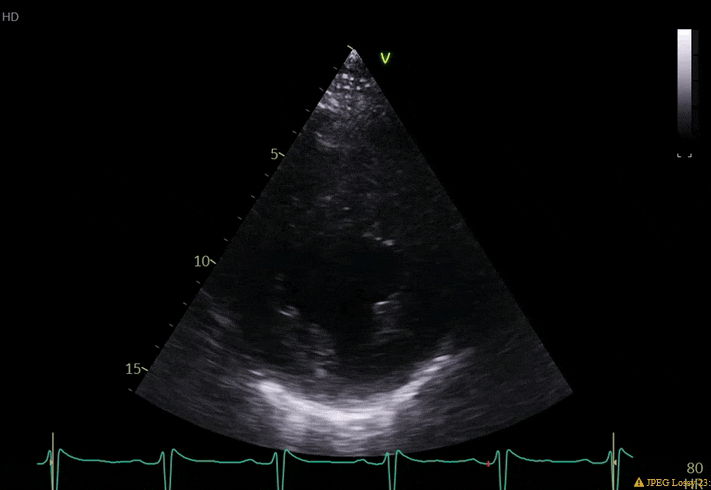
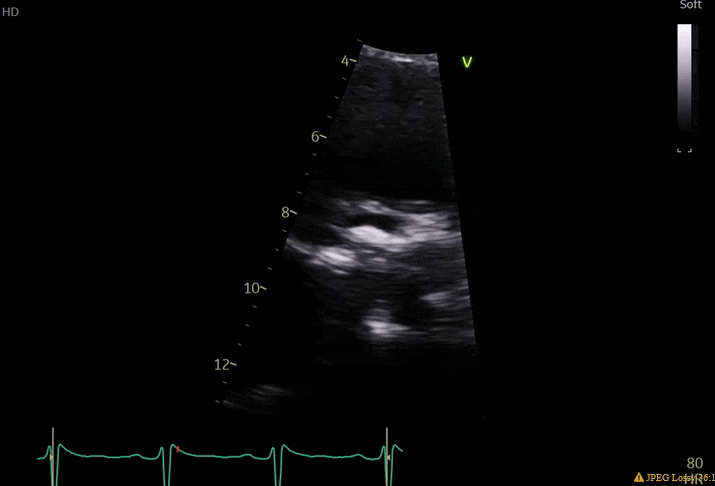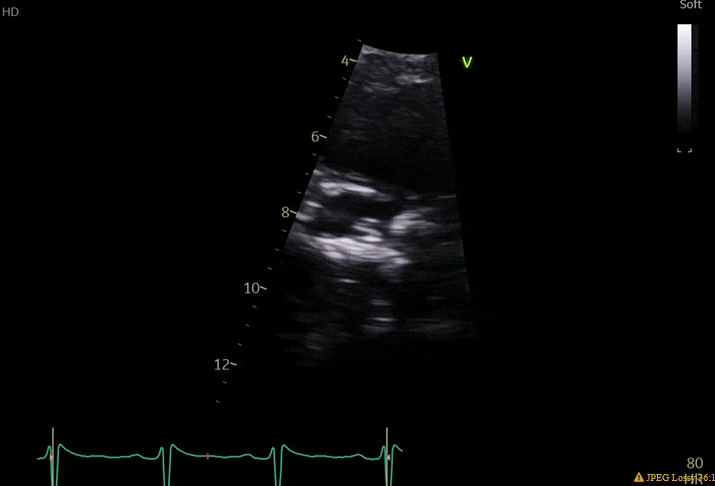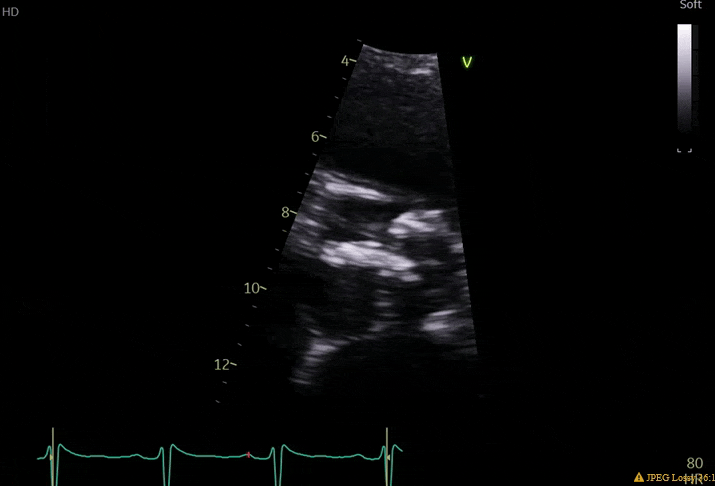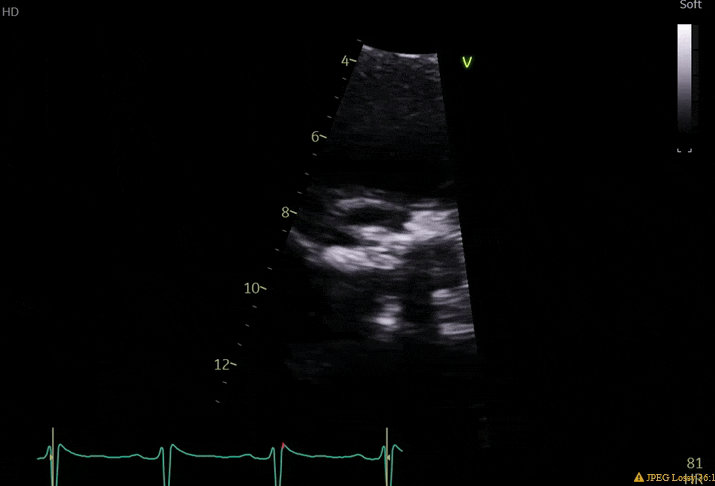
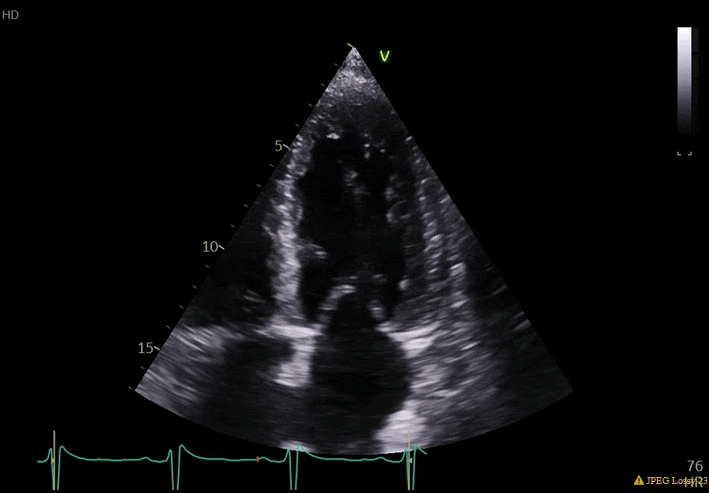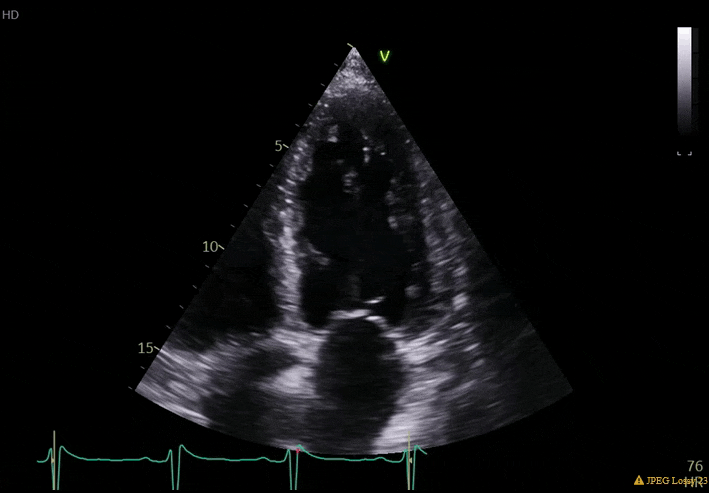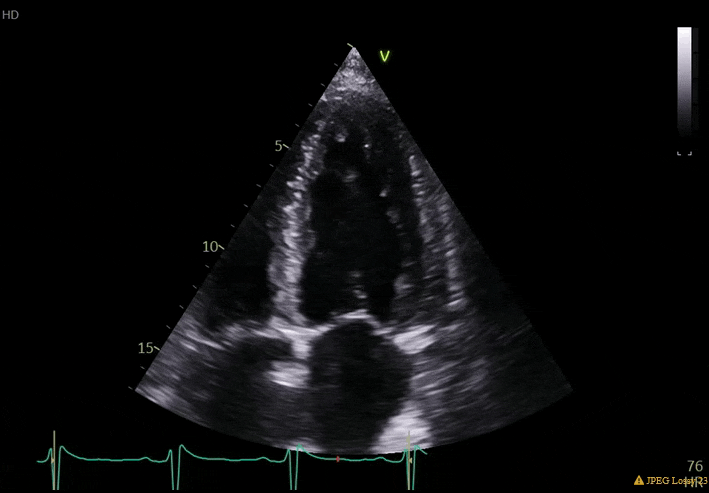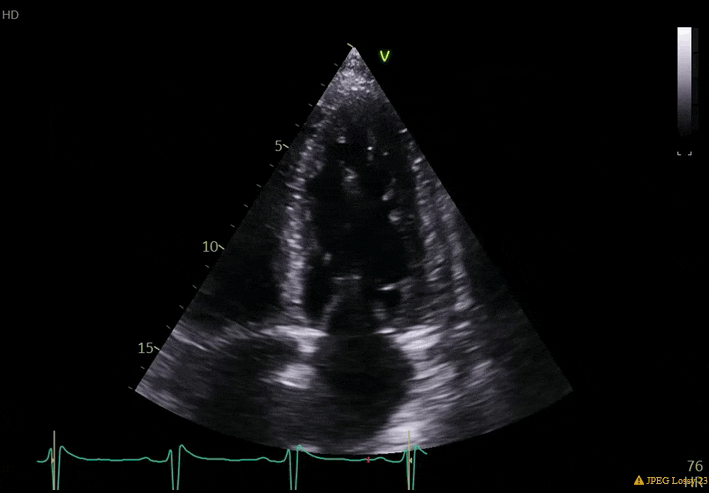
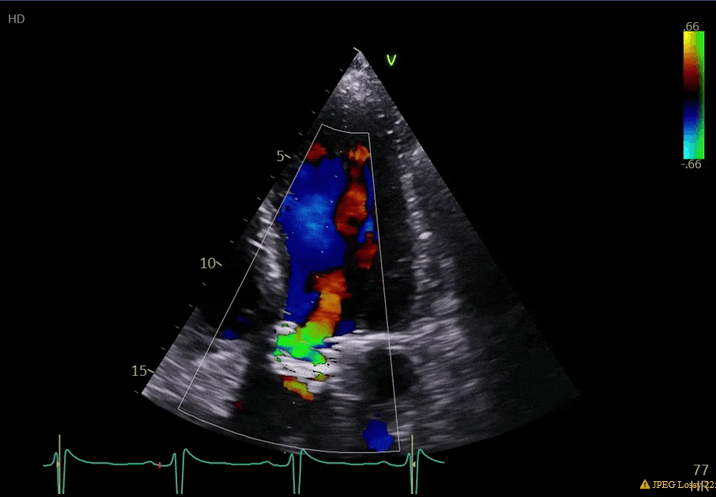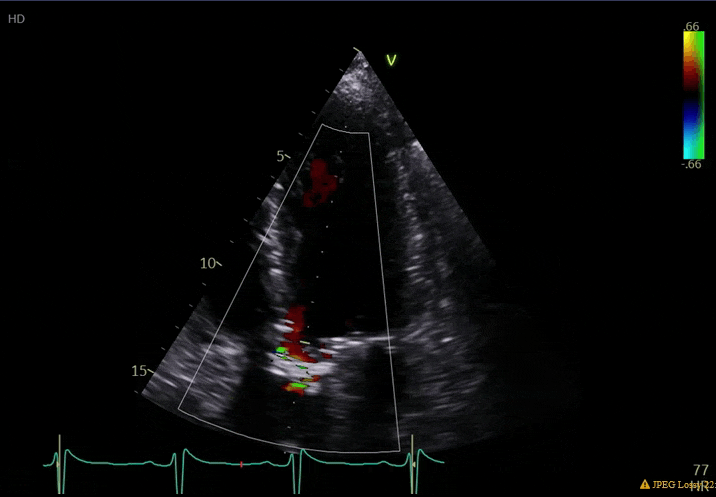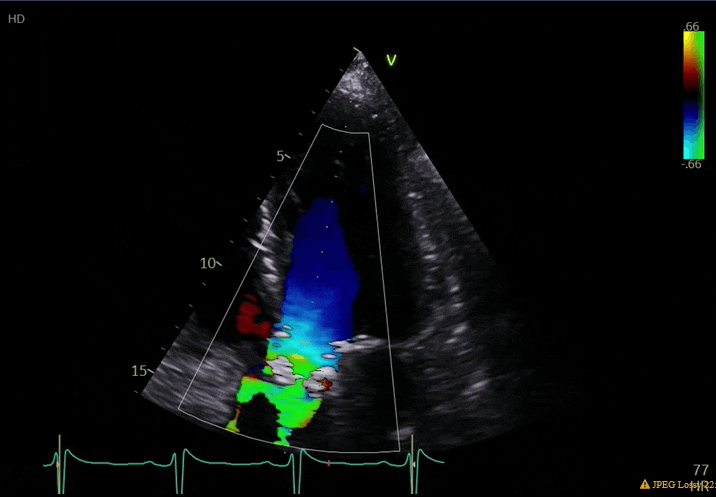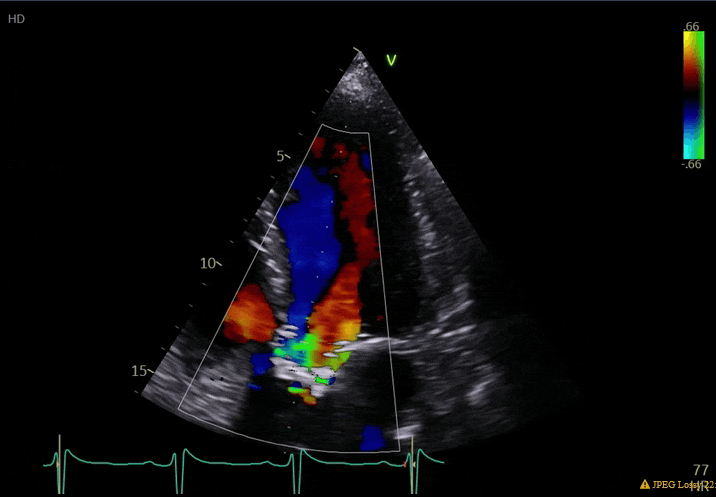
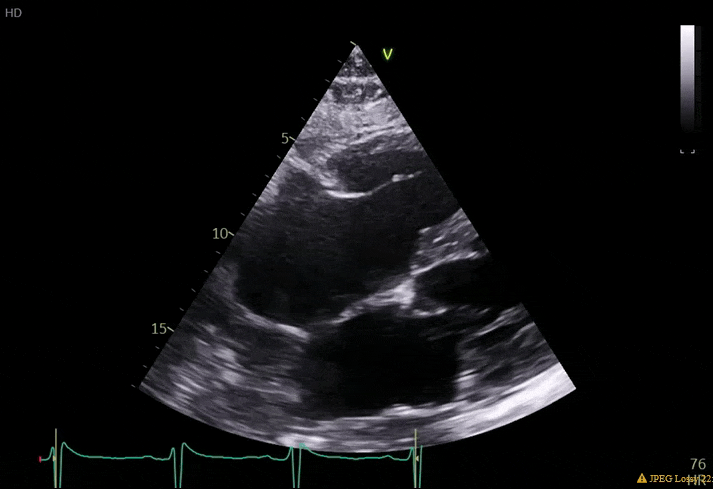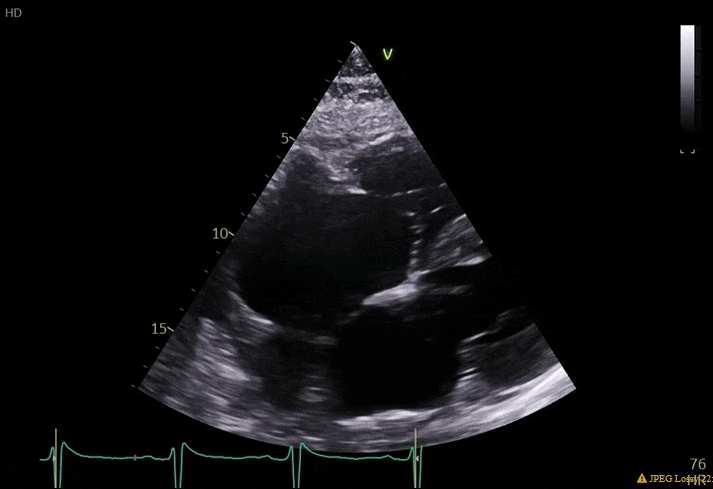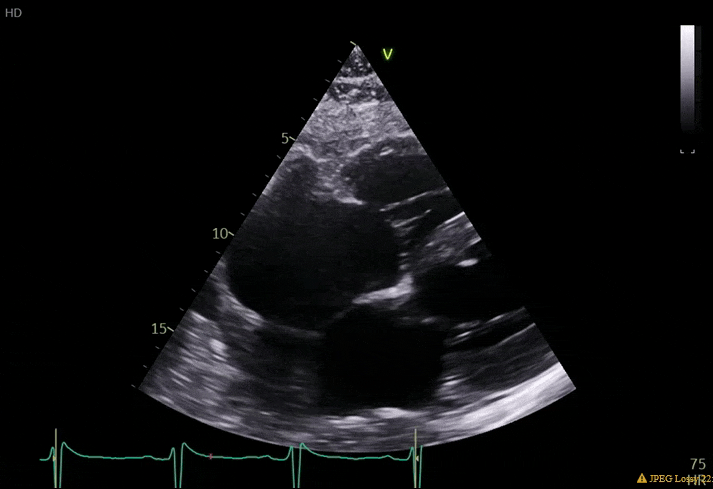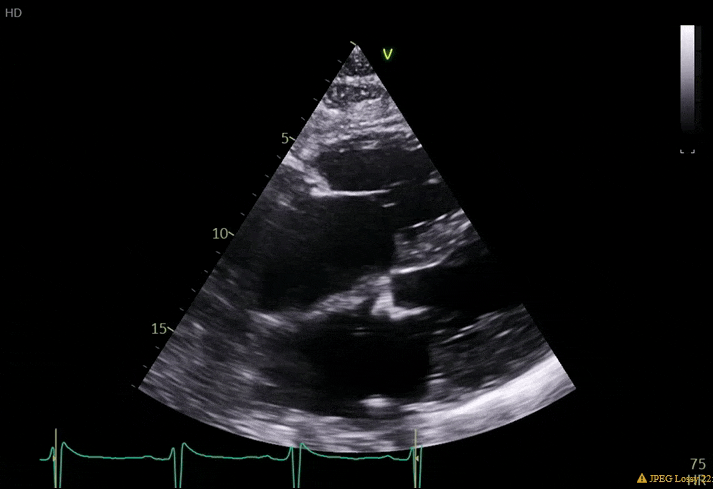
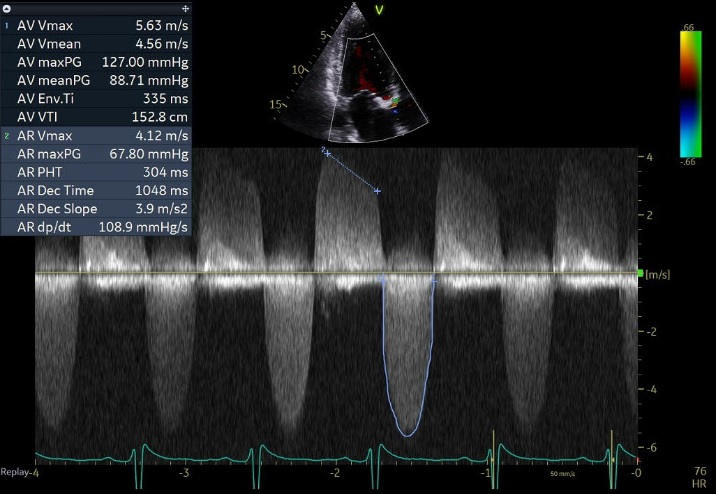
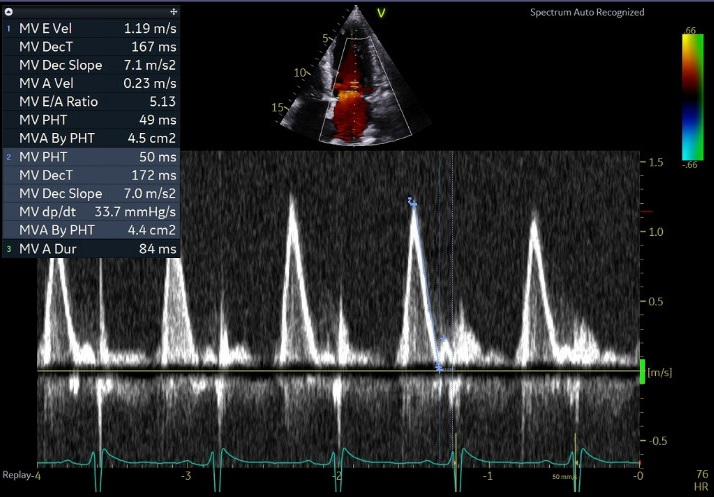
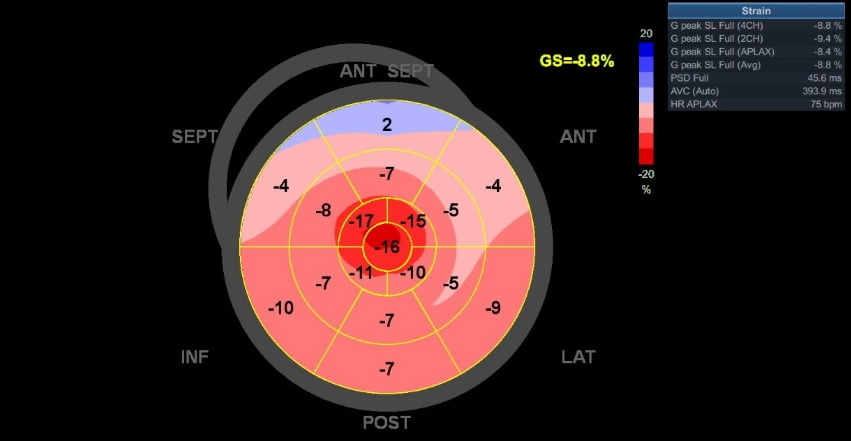
What else do you need to know? Any other workup? What specific inferences can be made from the mitral inflow, atria size, and strain pattern? What are our treatment options?
Here are some TTE instead of TEE images for a change.










What else do you need to know? Any other workup? What specific inferences can be made from the mitral inflow, atria size, and strain pattern? What are our treatment options?