- Joined
- May 17, 2008
- Messages
- 3,022
- Reaction score
- 3,617
Wanted to double check this one. The solution says there are 2 stereocenters (as marked with asterisks), but shouldn't the correct answer be 3, due to one of the double bonds?
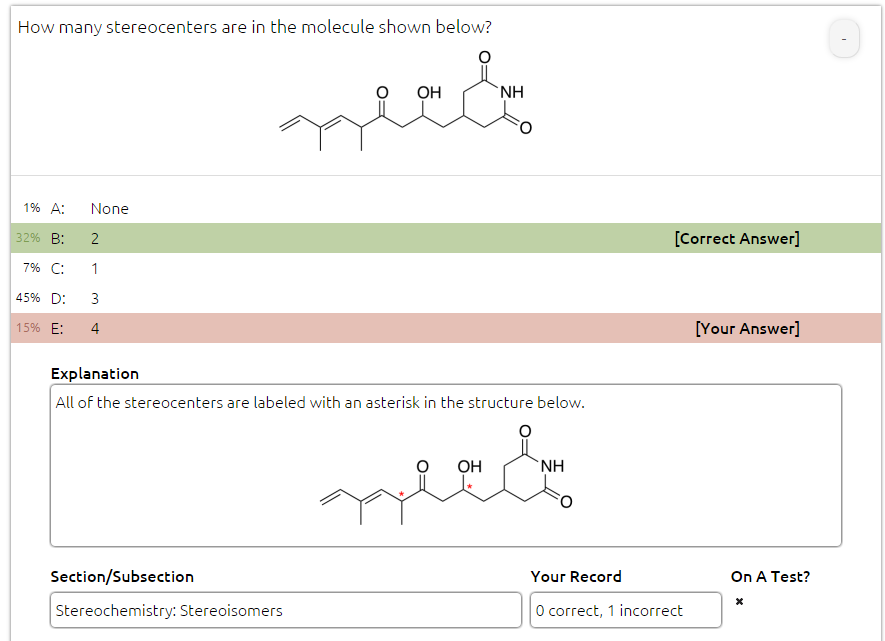


You said "A stereocenter means that by swapping the position of two groups, you would get a stereoisomer."
If we flip the C=C bond to make it from trans to cis, the two products are not mirror images, which is what stereoisomers are. These cis and trans molecules are diastereomers, which are not stereoisomers. Any carbon in an alkene bond cannot be a stereocenter.
Wanted to double check this one. The solution says there are 2 stereocenters (as marked with asterisks), but shouldn't the correct answer be 3, due to one of the double bonds?

