I am trying to practice more with graphs since that is my weakness.
So for the reaction rate versus time graph (below).
I know that Rate = -[Substrate]/time
[substrate] = rate x time
Is it correct to say that the area under the graph below is the [substrate]?
If the [substrate] is in excess, meaning that we have a huge abundance of the reactant or that we keep on replenishing it, would the graph just me a straight horizontal line?
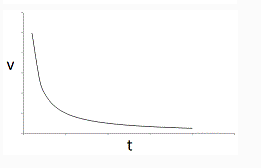
So for the reaction rate versus time graph (below).
I know that Rate = -[Substrate]/time
[substrate] = rate x time
Is it correct to say that the area under the graph below is the [substrate]?
If the [substrate] is in excess, meaning that we have a huge abundance of the reactant or that we keep on replenishing it, would the graph just me a straight horizontal line?
