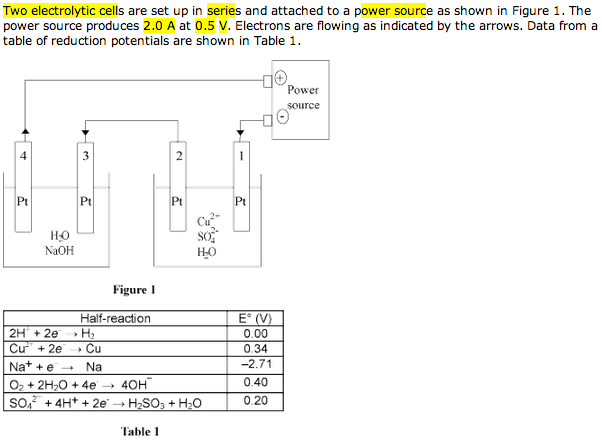
Well written post, but I'm having a hard time with your cell #2 process. I can't imagine you would need data that is not presented in the problem. The rest of it is solid.
...My Interpretation of why the table helps solve for electrode #2: (electrode 4 is more obvious)
Since you know the 2nd electrode is an anode from the given flow of electrons, then oxidation is occurring.
You also know that the 2nd cell is going to have a negative E* because
it was said that both cells are electrolytic.
Additionally the table gives you the reduction potential for the only possible electrode 1 reaction because there is only one cation in solution.
Electrode 1 reduction potential is Cu2+ 2e- >
Cu = 0.34
From the previous statement that
the cell is electrolytic you know that the
oxidation potential for the species at electrode 2 Must Be lower than -0.34, or else the cell would be galvanic.
If you only use the data from the table the possible Oxidation steps are from OH- or H2O+H2SO3 (might be assumed to be present)
To take the oxidation potential for (H2O+H2SO3) you could just take the negative of the reduction potential that is given (0.20). Giving you an oxidation potential of
-0.20.
Therefore you can eliminate that Oxidation potential of -0.2 because it is not negative enough.
The only option is the oxidation potential OH- > O2 + H20 + 2e- =
-0.4
That reaction would release Oxygen gas as expected by the answer.
Looking up the direct reduction potential for SO42- on Wiki gives the same answer.
SO42- + 4
H+ + 2
e−
SO2(
aq) + 2
H2O = +0.17
Oxidation potential would be -0.17 and not negative enough.
Source: http://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page)
Crazy question, I would have just educated guessed between A and C on a real MCAT.
And looking at the answer choices, three options have electrode 2 and three options have electrode 4, so it's probably both.