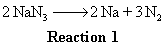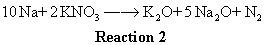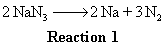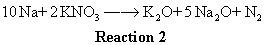This problem takes way more work than something they would put on the MCAT, but knowing stoichiometry really well is still useful sooo...
1st step is how many moles of gas do we want to produce? 45 L at STP is 2 moles of gas (close enough). OK, so for each mole of NaN3 we put in, how many moles of gas do we get? In other words, what is the overall stoichiometric relationship between moles of NaN3 supplied and moles of gas produced?
From reaction 1, 2 moles of NaN3 react to give 3 moles of N2, so 1 mole of NaN3 gives us 1.5 moles of gas from that reaction. But for each 1 mole of NaN3 that reacts, 1 moles of Na is produced (looking at the stoichiometric relationship from reaction 1). This 1 mole of Na product from reaction 1 will go on to yield 0.1 moles of N2 gas via reaction 2. Why? Since reaction 2 says for every 10 moles of Na you put in, you get one mole of N2 as a product. We will get 1/10 as many moles of gas product from reaction 2 as moles of Na reactant we put in.
Ok, so at this point we know we want 2 moles of gas to be produced and each mole of NaN3 we put in gives us 1.6 moles of N2 gas in total (1.5 moles via reaction 1 and 0.1 moles via reaction 2). Dividing the 2 moles of gas we want by the 1.6 moles of gas produced by each mole of NaN3 we put in tells us that we want to put in roughly 1.25 moles of NaN3. Multiplying 1.25 moles of NaN3 by its MW of 65 g/mol yields 81 g.
 76 grams
76 grams 81 grams
81 grams 87 grams
87 grams 130 grams
130 grams