- Joined
- Jan 16, 2009
- Messages
- 293
- Reaction score
- 0
9. Kinetic friction is seen when two objects are in contact, and neither of them are rotating (but one of them is sliding) while static friction ivolved one object rotating and the other is stationaey, correct?
Q: What type of friction is generated between the brake plate (which is attached to the wheel) and brake pad (which is stationary) as the car decelerated from its initial velocity, assuming that the wheels do not lock up?
Answer: kinetic friction only
The explanation says that this is because the brake plate is moving (rotating) Then wouldnt this be an example of static friction?
11. At high constant speed, what produces the MOST drag?
A. Kinetic friction
B. Static friction
C. Rolling friction
D. Air resistance
The explanation says that the force due to air resistant is proportional to the square of the velocity with which the vehicle is traveling. Where did this equation come from?
Also, I didnt know they were talking about a car One I know that, I guess its easy to rule out kinetic friction since there is none. They say to rule out static friction because its te reason that the car is moving, but howcome this isnt considered drag? And is force of rolling friction the same thing as static friction? Also, does air resistance produce the most drag for all moving objects or only for cars?
12. An antilock brake pad should be made of a material that:
A. maintains µs with increasing temperature.
</SPAN>B. increases µs with increasing temperature.
</SPAN>C. maintains µk with increasing temperature.
</SPAN>D. increases µk with increasing temperature.
</SPAN>
Why not increase? I know that in the passage it states that Antilock disk brake: Braking efficiency remains constant over the period of time that the brake is applied But if you increase µk you would increase friction, make the car slow down faster.
28. Assuming Theory 1 to be correct, the energy of a single photon could be determined from
Theory 1: e+ + e- = n + y (n = neutrino y = gamma ray)
A. ∆msystemc2
</SPAN>B. ∆msystemc2 + ½(∆mve+2 + ∆mve-2 - ∆mvη2)
</SPAN>C. ∆msystemc2 + ½(mve+2 + mve-2 - mvη2)
</SPAN>D. ∆msystemc2 + ½(∆pe+2 + ∆pe-2 + ∆pη2)
</SPAN>I do not understand the explanation for this. What exactly is mc^2 for?
30. The product of an electron-positron annihilation, according to Theory 2, must consist of two photons instead of just one in order to conserve:
A. energy
B. Charge
C. momentum
D. mass
Theory 2: e+ + e- à 2y
I picked momentum just because I saw the collision of 2 thing, was my reasoning valid here
38. Why do liquids/solids expand upon heating? I always thought of PV=nRT, but this only applies to gases
40. The MOST exothermic chemical reaction involves:
A: oxidation-reduction
Any other random facts I should know about this?
41. The MOST rapid temperature change would be observed with which system?
A. A 20.0-g pellet at 50°C added to 100 mL of water at 25°C.
</SPAN>B. A 20.0-g pellet at 70°C added to 100 mL of water at 25°C
</SPAN>
I know the answer intuitively, but whats the concept here?
47. According to Table 1, what properties can be expected for a 1.00-meter rod with a diameter of 2 mm that is made of an aluminum-copper alloy known to be 85% aluminum by moles mixed with copper?
A. It has a density of 3.63 g/mL and a resistance of 8.15 x 10-3 Ω.
</SPAN>B. It has a density of 3.63 g/mL and a resistance of 8.25 x 10-3 Ω.

So the way I did it is I saw 3.63 is closer to 20 and I picked A. How are you supposed to see that the mole percent of copper varies uniformly with both density and resistance??
51. In a double-slit experiment using a single light source passing through two slits, what is observed at a point on a luminescing screen that is 3 1/6 λ from the upper slit and 4 2/3 λ from the lower slit, as shown in the diagram below?
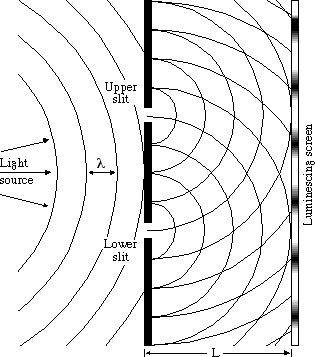 how do u do this?
how do u do this?
</SPAN>
Q: What type of friction is generated between the brake plate (which is attached to the wheel) and brake pad (which is stationary) as the car decelerated from its initial velocity, assuming that the wheels do not lock up?
Answer: kinetic friction only
The explanation says that this is because the brake plate is moving (rotating) Then wouldnt this be an example of static friction?
11. At high constant speed, what produces the MOST drag?
A. Kinetic friction
B. Static friction
C. Rolling friction
D. Air resistance
The explanation says that the force due to air resistant is proportional to the square of the velocity with which the vehicle is traveling. Where did this equation come from?
Also, I didnt know they were talking about a car One I know that, I guess its easy to rule out kinetic friction since there is none. They say to rule out static friction because its te reason that the car is moving, but howcome this isnt considered drag? And is force of rolling friction the same thing as static friction? Also, does air resistance produce the most drag for all moving objects or only for cars?
12. An antilock brake pad should be made of a material that:
A. maintains µs with increasing temperature.
</SPAN>B. increases µs with increasing temperature.
</SPAN>C. maintains µk with increasing temperature.
</SPAN>D. increases µk with increasing temperature.
</SPAN>
Why not increase? I know that in the passage it states that Antilock disk brake: Braking efficiency remains constant over the period of time that the brake is applied But if you increase µk you would increase friction, make the car slow down faster.
28. Assuming Theory 1 to be correct, the energy of a single photon could be determined from
Theory 1: e+ + e- = n + y (n = neutrino y = gamma ray)
A. ∆msystemc2
</SPAN>B. ∆msystemc2 + ½(∆mve+2 + ∆mve-2 - ∆mvη2)
</SPAN>C. ∆msystemc2 + ½(mve+2 + mve-2 - mvη2)
</SPAN>D. ∆msystemc2 + ½(∆pe+2 + ∆pe-2 + ∆pη2)
</SPAN>I do not understand the explanation for this. What exactly is mc^2 for?
30. The product of an electron-positron annihilation, according to Theory 2, must consist of two photons instead of just one in order to conserve:
A. energy
B. Charge
C. momentum
D. mass
Theory 2: e+ + e- à 2y
I picked momentum just because I saw the collision of 2 thing, was my reasoning valid here
38. Why do liquids/solids expand upon heating? I always thought of PV=nRT, but this only applies to gases
40. The MOST exothermic chemical reaction involves:
A: oxidation-reduction
Any other random facts I should know about this?
41. The MOST rapid temperature change would be observed with which system?
A. A 20.0-g pellet at 50°C added to 100 mL of water at 25°C.
</SPAN>B. A 20.0-g pellet at 70°C added to 100 mL of water at 25°C
</SPAN>
I know the answer intuitively, but whats the concept here?
47. According to Table 1, what properties can be expected for a 1.00-meter rod with a diameter of 2 mm that is made of an aluminum-copper alloy known to be 85% aluminum by moles mixed with copper?
A. It has a density of 3.63 g/mL and a resistance of 8.15 x 10-3 Ω.
</SPAN>B. It has a density of 3.63 g/mL and a resistance of 8.25 x 10-3 Ω.

So the way I did it is I saw 3.63 is closer to 20 and I picked A. How are you supposed to see that the mole percent of copper varies uniformly with both density and resistance??
51. In a double-slit experiment using a single light source passing through two slits, what is observed at a point on a luminescing screen that is 3 1/6 λ from the upper slit and 4 2/3 λ from the lower slit, as shown in the diagram below?

</SPAN>

