avalonisland888
Full Member
- Joined
- May 22, 2020
- Messages
- 60
- Reaction score
- 7
1) I'm not quite understanding the explanation for this question. They talk about how the Cl- ion can react with the Ag+ and how this can ultimately create more I- ions as NaCl concentrations increase while keeping the Ag+ concentrations low. I assumed that since there is no common ion effect, there shouldn't be any change at all.

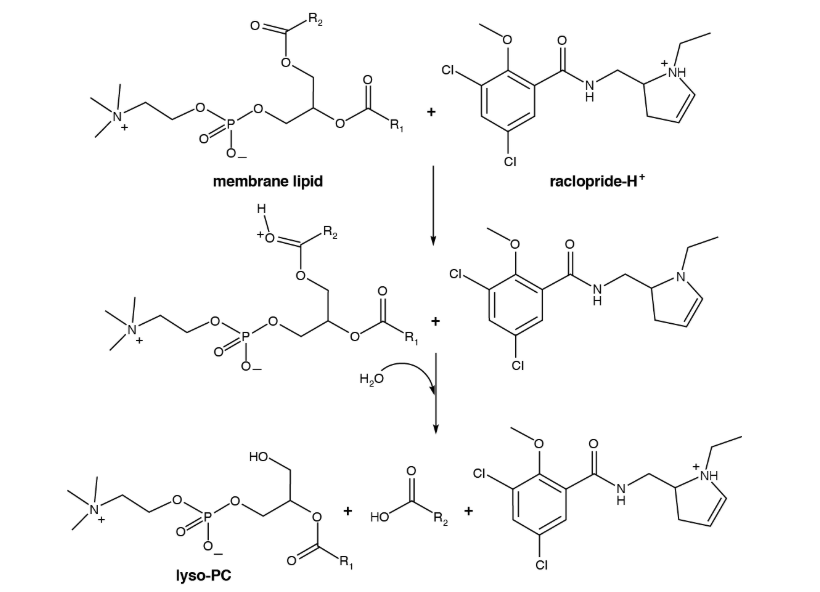
2) Why does the NH+ to N in raclopride-H+ not count as oxidation if we're losing the H?
3) Why are ATPases and GTPases hydrolases instead of oxidoreductases? The way I understand it is GTP/ATP gets reduced to ADP/GDP. I looked it up online and it does that ADP is the reduced form of ATP, but am I just interpreting this incorrectly since it's more of a hydrolysis and not a reduction? I think I'm getting confused with the enzyme categories because let's say for succinyl CoA synthetase. It's a ligase and not a hydrolase despite producing GTP. Is the difference because GTP/ATPases' substrate IS the ATP/GTP whereas for the synthetases and dehydrogenases, their substrate is something else but they PRODUCE the energy?
2) Why does the NH+ to N in raclopride-H+ not count as oxidation if we're losing the H?
3) Why are ATPases and GTPases hydrolases instead of oxidoreductases? The way I understand it is GTP/ATP gets reduced to ADP/GDP. I looked it up online and it does that ADP is the reduced form of ATP, but am I just interpreting this incorrectly since it's more of a hydrolysis and not a reduction? I think I'm getting confused with the enzyme categories because let's say for succinyl CoA synthetase. It's a ligase and not a hydrolase despite producing GTP. Is the difference because GTP/ATPases' substrate IS the ATP/GTP whereas for the synthetases and dehydrogenases, their substrate is something else but they PRODUCE the energy?