- Joined
- Aug 12, 2009
- Messages
- 581
- Reaction score
- 83
EK1001 Ochem #631:
Which of the following would be most acidic
A. Ethanol
B. Benzophenone
C. Methyl benzoate
D. Triphenylcarbinol
Structure of D:
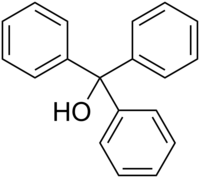
The solution says that D is most acidic b/c "it has extensive resonance stabilisation of the conjugate base's negative charge".
This seems strange to me because unlike in the case of phenol, the anionic charge would not be at a benzylic position. In addition, wouldn't the three phenyls, by virtue of high electron density, stabilize a slight partial positive charge at the benzylic carbon, such that the anion is actually disfavored? Isn't this why phenylmethanol has pretty much the same pKa as methanol?
Of course once I tried to corroborate this with real data things got really complicated:
According to wikipedia, the pka of D is "16-18".
Yet the literature seems to suggest it's actually a strong acid with a pka of -6.6:
http://onlinelibrary.wiley.com/doi/10.1002/jctb.503310162/pdf
http://www.sciencedirect.com/science/article/pii/0021951765900941
Or in some cases, 12.7
http://www.jstage.jst.go.jp/article/bcsj/84/1/82/_pdf
http://www.chemicalbook.com/ProductMSDSDetailCB3335936_EN.htm
But definitely lower than ethanol, which is about 16.
I am utterly confused...
Which of the following would be most acidic
A. Ethanol
B. Benzophenone
C. Methyl benzoate
D. Triphenylcarbinol
Structure of D:

The solution says that D is most acidic b/c "it has extensive resonance stabilisation of the conjugate base's negative charge".
This seems strange to me because unlike in the case of phenol, the anionic charge would not be at a benzylic position. In addition, wouldn't the three phenyls, by virtue of high electron density, stabilize a slight partial positive charge at the benzylic carbon, such that the anion is actually disfavored? Isn't this why phenylmethanol has pretty much the same pKa as methanol?
Of course once I tried to corroborate this with real data things got really complicated:
According to wikipedia, the pka of D is "16-18".
Yet the literature seems to suggest it's actually a strong acid with a pka of -6.6:
http://onlinelibrary.wiley.com/doi/10.1002/jctb.503310162/pdf
http://www.sciencedirect.com/science/article/pii/0021951765900941
Or in some cases, 12.7
http://www.jstage.jst.go.jp/article/bcsj/84/1/82/_pdf
http://www.chemicalbook.com/ProductMSDSDetailCB3335936_EN.htm
But definitely lower than ethanol, which is about 16.
I am utterly confused...
