- Joined
- Jun 11, 2009
- Messages
- 606
- Reaction score
- 0
I understand this question may be a bit unusual for the clinician forum; however, I am not premed nor am I studying for any examination such as DAT, MCAT and so on, therefore I am not exactly sure where to put this question. I simply think about concepts for the sake of pure mental masturbation. Moderators, please feel free to move and my apologies if this creates a problem.
I have been experiencing a dilemma and simply cannot wrap my head around two concepts that are creating a dichotomy in my brain and frankly, driving me nuts.
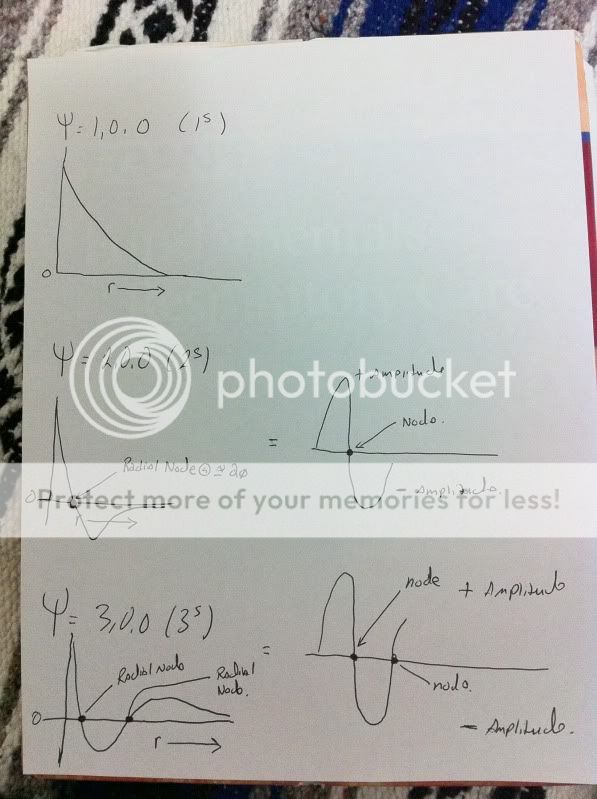
So, when looking at the solutions of the Schrodinger equation (assuming a hydrogen atom), I am having difficulty interpreting the physical meaning of nodes such as radial nodes.
For example, say I have solve the Schrodinger equation for a 2s wavefunction, square the value, obtain a probability density, and so on. So, when we do all this nifty stuff and graph the square of the wavefunction, we have a node where we have zero probability density of finding an electron. I think this node lives somewhere around r~ 1 Angstrom ( two times the Bohr radius).
My dilemma is this:
I can interpret this to say that I will "never" find an electron at this distance (r). However, this appears highly deterministic and I have always though in spite of having an area of very low probability, the electron could be found, well anywhere. It would just be highly improbable to find it certain areas. Yet, here I have something that says, you can "never" find it at a node.
My problem is making sense of what appears to be somewhat of a contradiction. Clearly, I am missing something here and would appreciate any guidance anybody has to offer.
I have been experiencing a dilemma and simply cannot wrap my head around two concepts that are creating a dichotomy in my brain and frankly, driving me nuts.
So, when looking at the solutions of the Schrodinger equation (assuming a hydrogen atom), I am having difficulty interpreting the physical meaning of nodes such as radial nodes.
For example, say I have solve the Schrodinger equation for a 2s wavefunction, square the value, obtain a probability density, and so on. So, when we do all this nifty stuff and graph the square of the wavefunction, we have a node where we have zero probability density of finding an electron. I think this node lives somewhere around r~ 1 Angstrom ( two times the Bohr radius).
My dilemma is this:
I can interpret this to say that I will "never" find an electron at this distance (r). However, this appears highly deterministic and I have always though in spite of having an area of very low probability, the electron could be found, well anywhere. It would just be highly improbable to find it certain areas. Yet, here I have something that says, you can "never" find it at a node.
My problem is making sense of what appears to be somewhat of a contradiction. Clearly, I am missing something here and would appreciate any guidance anybody has to offer.



 OP your screen name sticks out to me.
OP your screen name sticks out to me.