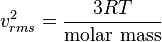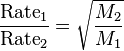- Joined
- Dec 26, 2006
- Messages
- 951
- Reaction score
- 0
Roman Numeral Question
I. Molecular Mass
II. Temperature
III. Pressure
I said Temperature and Pressure. Don't have the answer
I reasoned... Temperature gives the particles more kinetic energy... and decreasing pressure.. makes them collide with each other more (faster.. maybe. not sure)
I thought Molecular mass did not play a role b/c aren't we told that all gases behave the same way? That's why we have the ideal gas law?
I. Molecular Mass
II. Temperature
III. Pressure
I said Temperature and Pressure. Don't have the answer
I reasoned... Temperature gives the particles more kinetic energy... and decreasing pressure.. makes them collide with each other more (faster.. maybe. not sure)
I thought Molecular mass did not play a role b/c aren't we told that all gases behave the same way? That's why we have the ideal gas law?