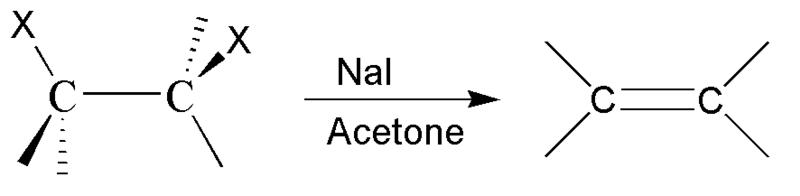
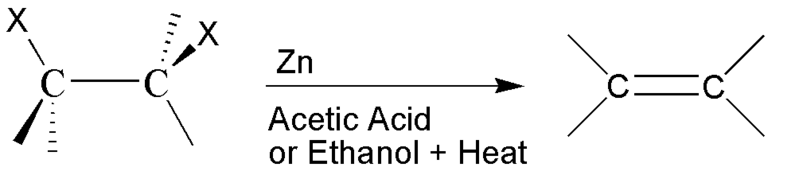
The question is: What alkene is obtained from this following reaction?
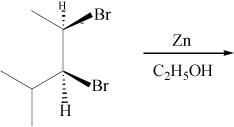
A. trans-4-methyl-2-pentene Kaplan says: "The bromides must be vicinal leading to a trans product, not cis." What?
B. cis-4-methyl-2-pentene
C. 4-methyl-1-pentene
D. mixture of trans-4-methyl-2-pentene and cis-4-methyl-2-pentene And why is there not a mixture?
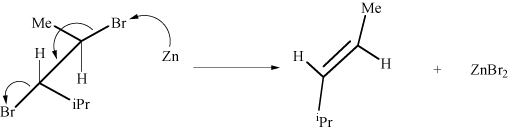

A. trans-4-methyl-2-pentene Kaplan says: "The bromides must be vicinal leading to a trans product, not cis." What?
B. cis-4-methyl-2-pentene
C. 4-methyl-1-pentene
D. mixture of trans-4-methyl-2-pentene and cis-4-methyl-2-pentene And why is there not a mixture?

Last edited: