- Joined
- Aug 6, 2007
- Messages
- 553
- Reaction score
- 5
I was taking a practice gen chem exam from my class a couple years ago, and came across a few questions I just couldn't figure out. Take a look...
1. A 151 g sample of a metal, M, reacts with exactly .35 moles of oxygen to form a metal oxide with the formula M2O. What is the identity of M?
a. Cs
b. Na
c. Au
d. Rb
e. Ag
2. How many electrons in a given atom can be described using the following quantum numbers? n=4, l=2, ml=2
a. 2
b. 3
c. 5
d. 6
e. 10
3. Determine the ground state electron configuration for Cr3+.
a. [Ar]4s2,3d1
b. [Ar]
c. [Ar]3d3
d. [Ar]4s2,3d7
e. [Ar]3d9
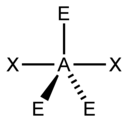
4. Is XeF2 a polar molecule? (I was just curious about this one)
Answers:
1. e
2. a
3. c
1. A 151 g sample of a metal, M, reacts with exactly .35 moles of oxygen to form a metal oxide with the formula M2O. What is the identity of M?
a. Cs
b. Na
c. Au
d. Rb
e. Ag
2. How many electrons in a given atom can be described using the following quantum numbers? n=4, l=2, ml=2
a. 2
b. 3
c. 5
d. 6
e. 10
3. Determine the ground state electron configuration for Cr3+.
a. [Ar]4s2,3d1
b. [Ar]
c. [Ar]3d3
d. [Ar]4s2,3d7
e. [Ar]3d9
4. Is XeF2 a polar molecule? (I was just curious about this one)
Answers:
1. e
2. a
3. c