- Joined
- Sep 12, 2011
- Messages
- 431
- Reaction score
- 34
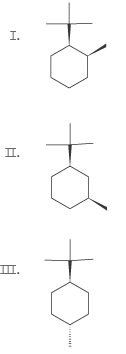
I'm a little hazy on drawing chair conformations and after trying to refresh myself, I'm still having some issues with this. Can someone explain how to figure out what the chair conformations of each of these are supposed to look like?
I think I'm having trouble figuring out how cis/trans and axial/equatorial correlate.
Thank you
I think I'm having trouble figuring out how cis/trans and axial/equatorial correlate.

Thank you