- Joined
- Mar 18, 2013
- Messages
- 11
- Reaction score
- 1
Hey all,
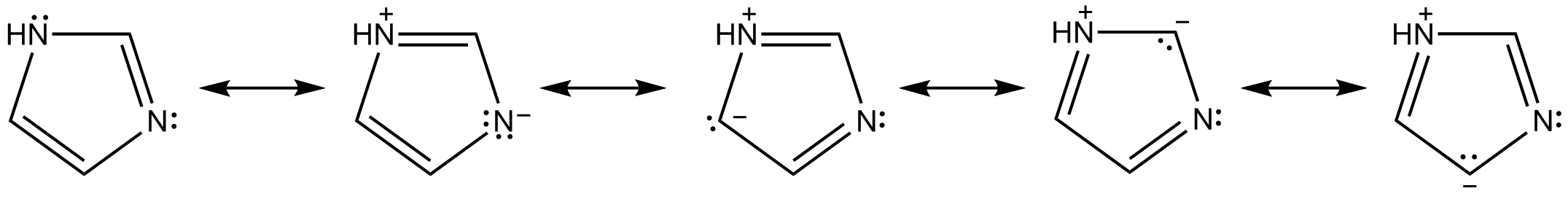
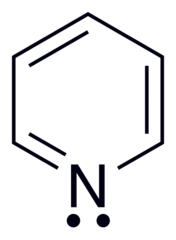
I am a little confused as to why the lone pair on the nitrogen molecule in pyridine is considered localized instead of a delocalized. I have it drawn where the pi electrons are pushed on nitrogen, and the lone pair from nitrogen pushed into the ring/pi system, hence stating the lone pair is delocalized. This seems more of a organic arrow pushing related question but it's bugging the crap out of me...
...is the rate of pi electron conjugation above and below the ring supersede the lone pair contributing to resonance?
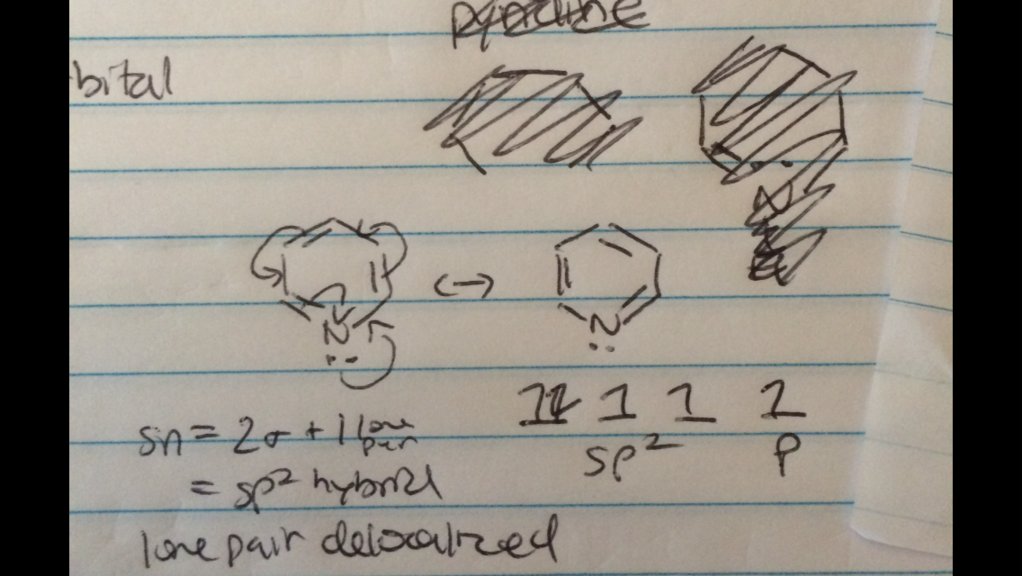
I am a little confused as to why the lone pair on the nitrogen molecule in pyridine is considered localized instead of a delocalized. I have it drawn where the pi electrons are pushed on nitrogen, and the lone pair from nitrogen pushed into the ring/pi system, hence stating the lone pair is delocalized. This seems more of a organic arrow pushing related question but it's bugging the crap out of me...
...is the rate of pi electron conjugation above and below the ring supersede the lone pair contributing to resonance?