- Joined
- Dec 18, 2015
- Messages
- 3,216
- Reaction score
- 4,930
Post-lump breast RT done in 5 fractions... old and busted.
But now, one and done (in the OR), we can confidently say. (Which takes us from about 3 patients/day on beam for breast to ~0).
Discuss.
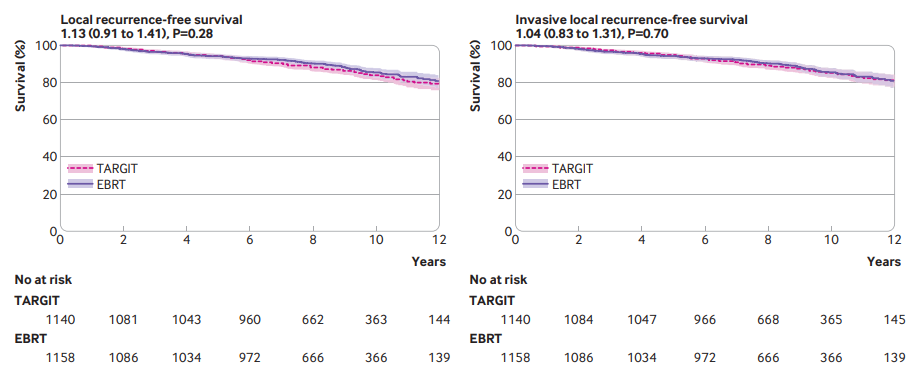
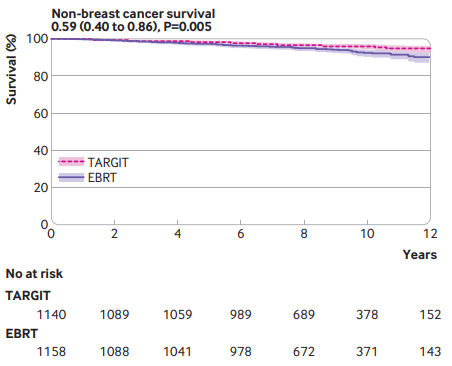
"For patients with early breast cancer who met our trial selection criteria (T1-2 [0-3.5cm]/N0-1, 45y+, IORT N=1140, EBRT N=1158; ~9-19y followup), risk adapted immediate single dose TARGIT-IORT during lumpectomy was an effective alternative to EBRT, with comparable long term efficacy for cancer control and lower non-breast cancer mortality (0.59, 0.40 to 0.86, P=0.005). TARGIT-IORT should be discussed with eligible patients when breast conserving surgery is planned."
But now, one and done (in the OR), we can confidently say. (Which takes us from about 3 patients/day on beam for breast to ~0).
Discuss.
"For patients with early breast cancer who met our trial selection criteria (T1-2 [0-3.5cm]/N0-1, 45y+, IORT N=1140, EBRT N=1158; ~9-19y followup), risk adapted immediate single dose TARGIT-IORT during lumpectomy was an effective alternative to EBRT, with comparable long term efficacy for cancer control and lower non-breast cancer mortality (0.59, 0.40 to 0.86, P=0.005). TARGIT-IORT should be discussed with eligible patients when breast conserving surgery is planned."