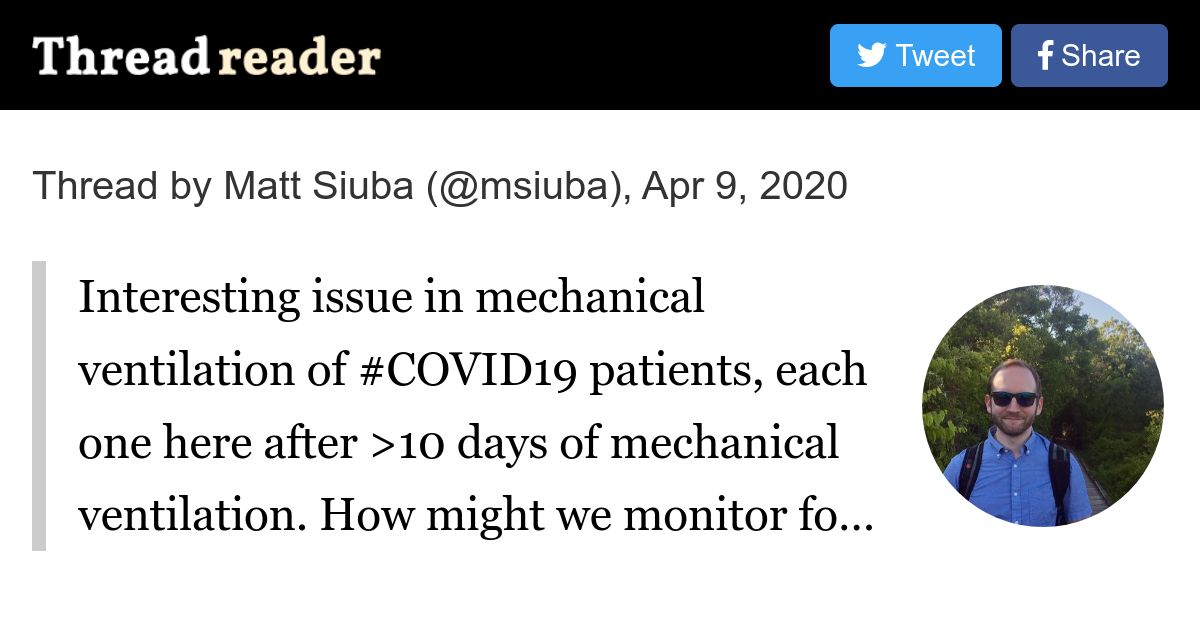
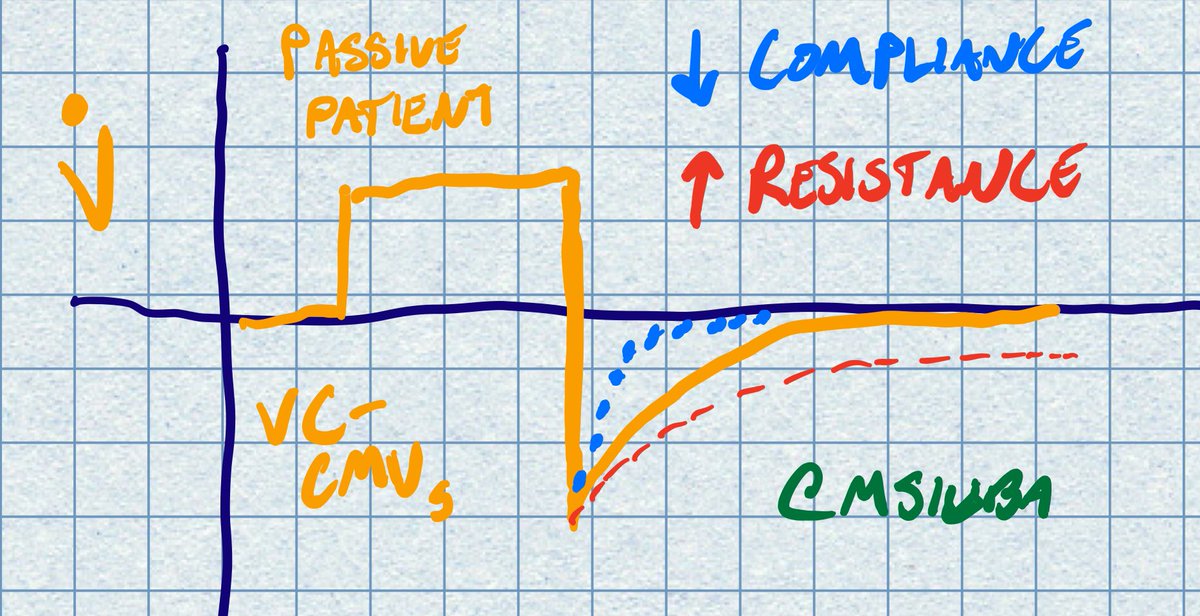
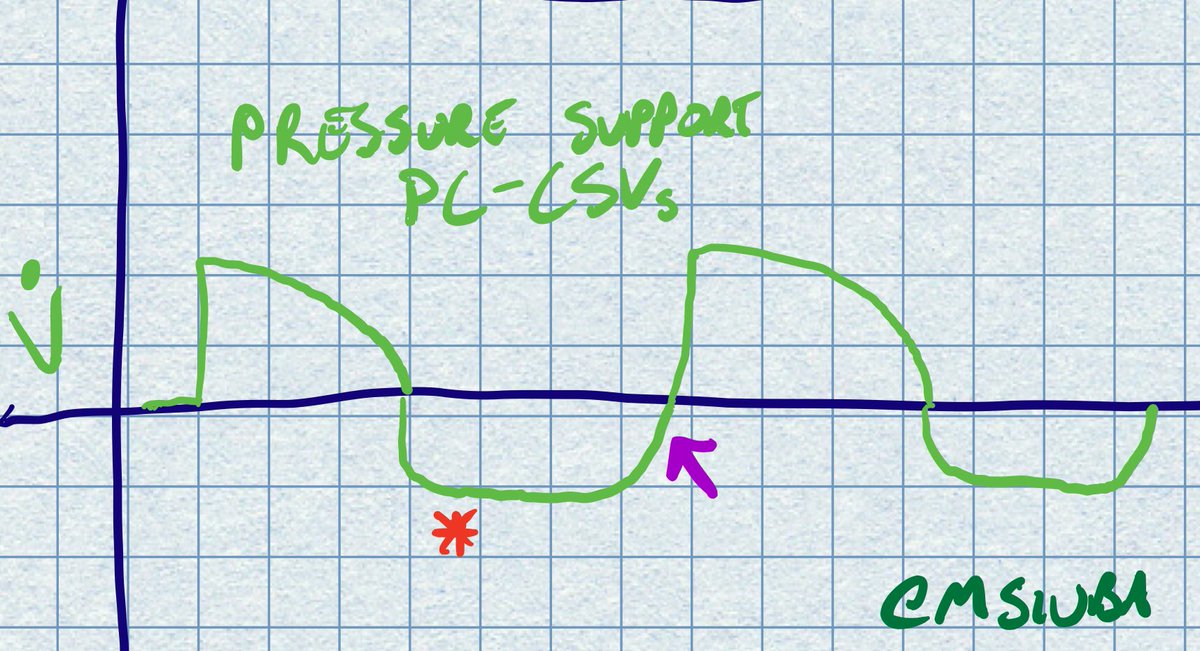
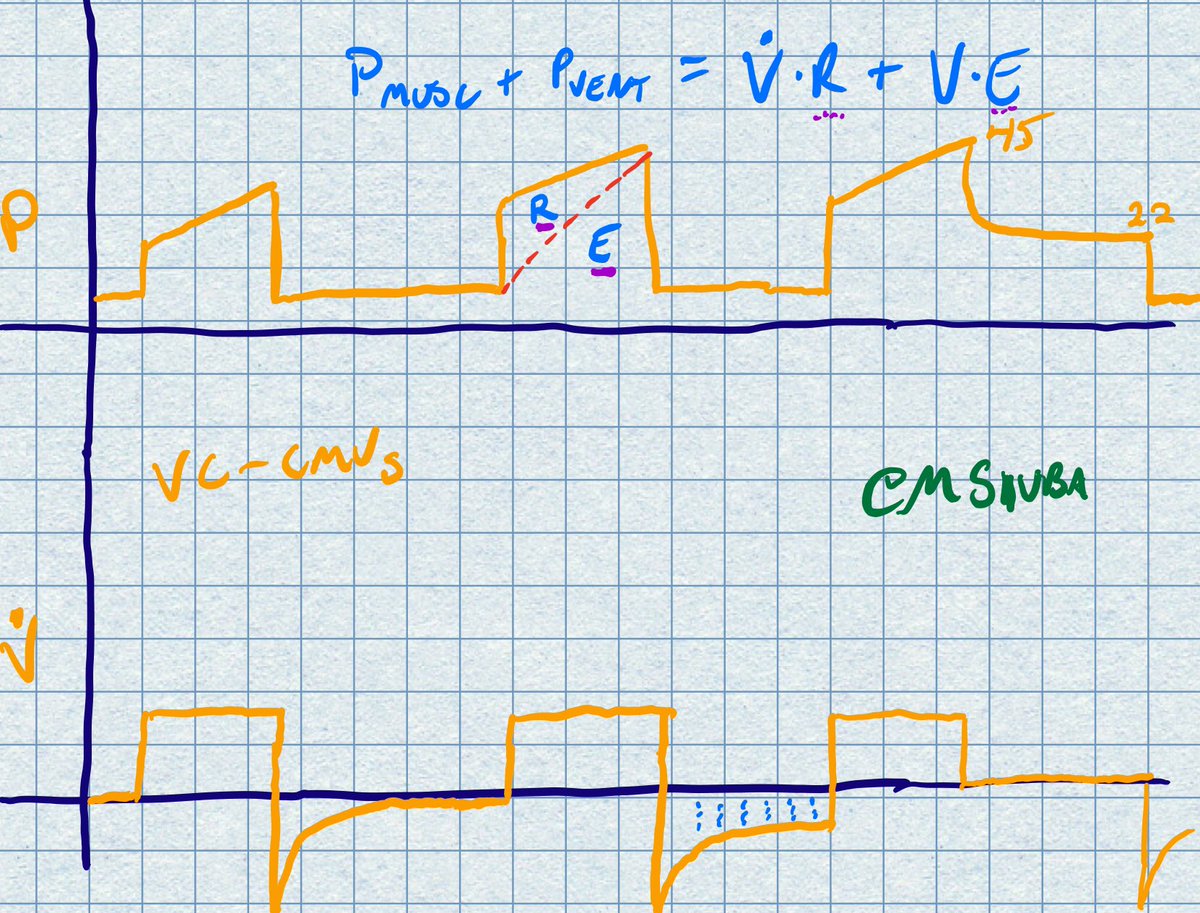
Right, as in, if the paO2 doesnt require 100% fi02 , why are they prone at all? I would think that I would reserve turning prone (and all the complications that go with it) for those who have no other option, they are already maxed out on other oxygenation efforts..
can you provide evidence that it reduces mortality?
From what I have seen the evidence of reduced mortality is marginal even in a study with questionable methods
Here is an interesting one: Prone position for acute respiratory failure in adults. - PubMed - NCBI
And also, Im pretty sure that If i tried as hard as the guys who do most ICU studies, I could eek out a study that showed marginal benefit to recruitment manuevers and bronchodilators... or almost any intervention that i chose to study
I cant tell you how many times I have a patient with respiratory distress, no asthma history (but just had an LMA or a ETT in place) low sats, someone says "they are not wheezing" you give albuterol and they feel better and sats improve..
I think beta agonists are being underused from what Im seeing for that exact reasoning. You don't have to necessarily have a history of asthma or acute bronchospasm to get some oxygenation benefit from albuterol, especially when you have a viral infection of your airway causing some degree of reactivity.
All fair points. You could argue that inhaled beta agonists are acting as inhaled pulmonary vasodilators.
I think that I see plenty of improvement in people with proning and that I wouldn’t want to wait till they’re at 100% to do it. I think also that different interventions are of benefit in a viral pneumonitis that will take weeks to resolve vs a theatre case that you expect to extubate at the end (or at least the next day).
Absolutely. I try not to go further than I'd like people to go for me (and I hate even the idea of being inpatient, not to mention the ICU). To me, every day a patient spends in most ICUs is torture (just the noise the nurses and their lazy alarms make should be unacceptable).
But I've seen enough turnarounds to be less pessimistic than people who underestimate the power of iatrogenic ****-ups in their differential diagnoses. As long as one is not too late. Unfortunately, there is badness caused by disease, and badness caused by bad treatments.
Agreed. For example, over peeping. IMHO it is the rare person that really needs (or doesn’t do worse on) 20 of peep