Phosphate buffered saline (PBS) is composed of various salts, including NaCl, KCl, and Na2HPO4. The highest measured current at a given voltage during the experiment would most likely be produced by PBS with which mass ratio of these salts, respectively?
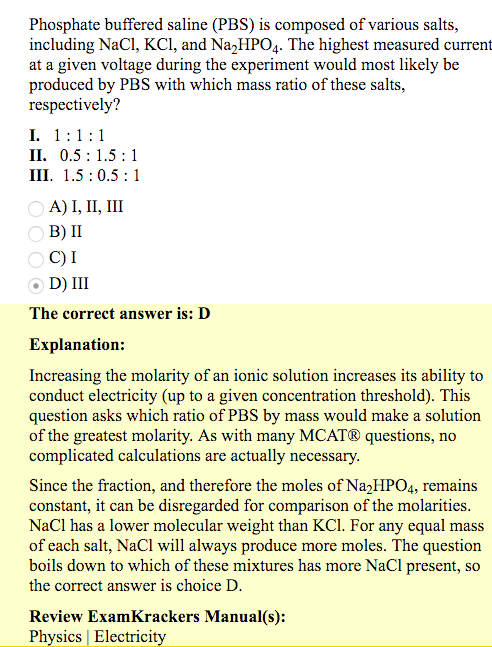
Not really understanding this explanation. Why does the "fraction, and therefore the moles of Na2HPO4" remain constant? Does it have to do with it not dissociating fully like NaCl or KCl? That's why we can ignore Na2HPO4?
Not really understanding this explanation. Why does the "fraction, and therefore the moles of Na2HPO4" remain constant? Does it have to do with it not dissociating fully like NaCl or KCl? That's why we can ignore Na2HPO4?
