- Joined
- May 17, 2008
- Messages
- 3,022
- Reaction score
- 3,617
I'm having a bit of an issue with this Qvault question on enthalpy:
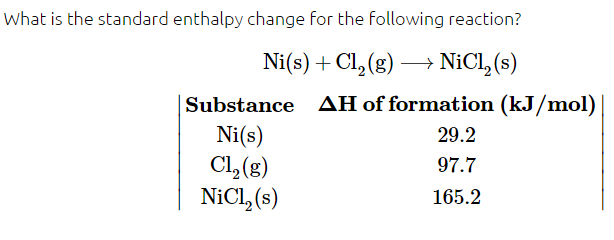
According to Chad, any element in its standard state, will have a ΔHf of 0 by definition. That would lead me to think the ΔHf of this reaction is just 165.2. According to Qvault: "The enthalpy change is represented by the following equation ΔH rxn = ΔH products - ΔH reactants. So the answer is ΔH=(165.2)−(29.2+97.7). This is equal to 38.3 kJ/mol"
I understand the math they used, but why do Ni and Cl2 (being in their standard states) not have a ΔHf of 0?

According to Chad, any element in its standard state, will have a ΔHf of 0 by definition. That would lead me to think the ΔHf of this reaction is just 165.2. According to Qvault: "The enthalpy change is represented by the following equation ΔH rxn = ΔH products - ΔH reactants. So the answer is ΔH=(165.2)−(29.2+97.7). This is equal to 38.3 kJ/mol"
I understand the math they used, but why do Ni and Cl2 (being in their standard states) not have a ΔHf of 0?
