- Joined
- Jan 5, 2015
- Messages
- 146
- Reaction score
- 38
Can someone please help me out with this problem. Not sure where to start to figure this out. The correct answer is E.
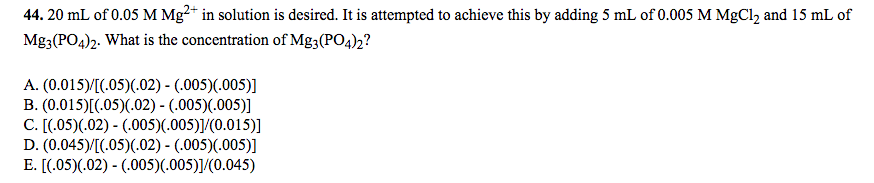
The answer is E.... here is an explanation for it but it's still not clear to me.I'm not sure, but I think the correct answer is C
Okay that clears a lot up for me but I don't understand why are we subtracting Mg2+ moles at the end? The question asks for the concentration of Mg3(PO4)2, isn't M2 fromThats the most complicated explanation ever.
This is how I solve it. When looking for the conc of Mg3(PO4)2, use this formula N1M1V1=N2M2V2. N=normality. Mg3(PO4)2 has a normality of 3. so
(.005 L)(.005 M MgCl2)= (3)(M2)(.015L) You solve for M2. That give you the concentration of Mg3(PO4)2. Then you just subtract the mole amount of Mg2+ from the concentration found above.
Basically
mole Mg2+ - ((N1M1V2/N2V2)
