- Joined
- May 10, 2008
- Messages
- 91
- Reaction score
- 0
Hi guys,
I was wondering if somebody could help me with the nmr problems that are usually on the DATs. Problems that ask number of peaks or how many singlets and triplets etc.
For example, can somebody walk me through this problem:
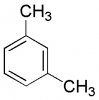
The h'nmr of m-xylene will show:
The answer is 4 peaks with 2 singlets and 1 doublet and 1 triplet....
Also, does a singlet have one or two adjacent hydrogens?
Thanks
I was wondering if somebody could help me with the nmr problems that are usually on the DATs. Problems that ask number of peaks or how many singlets and triplets etc.
For example, can somebody walk me through this problem:
The h'nmr of m-xylene will show:
The answer is 4 peaks with 2 singlets and 1 doublet and 1 triplet....
Also, does a singlet have one or two adjacent hydrogens?
Thanks
Last edited:

 Lol, yeah man. I already guessed that you have not counted the hydrogen attached to the C1. I'm sure you won't make this mistake on the real thing if you get one of these [I doubt though, has there ever been any of these type of problems on DAT?]. I mean it is still enjoyable to do them, but do they show up on the real thing?
Lol, yeah man. I already guessed that you have not counted the hydrogen attached to the C1. I'm sure you won't make this mistake on the real thing if you get one of these [I doubt though, has there ever been any of these type of problems on DAT?]. I mean it is still enjoyable to do them, but do they show up on the real thing?