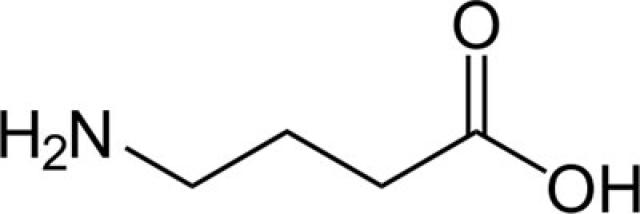
It helps to visualize the structure of GABA:
Which looks similar to the structure of glycine:
In both cases, these amino acids are diprotic and have a titration curve that looks something like this:
Henderson-Hasselbalch equation is useful for estimating the pH of a buffer solution and calculating the [base]/[acid] ratios of a weak acid-conjugate base pair. In diprotic amino acids, there are two buffer systems involved: protonated/zwitterion and zwitterion/deprotonated, where the zwitterion has an amphoteric property of being both the conjugate base of the protonated form and the conjugate acid of deprotonated form. So using the buffer equation, we see:
pH = pKa1 + log([zwitterion]/[protonated]);
pH = pKa2 + log([deprotonated]/[zwitterion]);
Note if pH = pKa1, log([zwitterion]/[protonated]) = 0 and [zwitterion]/[protonated] = 1. And similarly, if pH = pKa2, [deprotonated]/[zwitterion] = 1.
The isoelectric point pI is the pH at which the amino acid is electrically neutral. For a diprotic amino acid like GABA, pI = 1/2 * (pKa1 + pKa2). You can see from the titration curve that the pI is at OH- concentration where the protonated form is deprotonated entirely into zwitterion form, meaning that only the zwitterion form exists at pI, which ensures electrical neutrality. Past the pI and into pKa2, the amino acid develops a net negative charge as the zwitterion form gradually changes into deprotonated form until they reach equal concentrations at pKa2.
So the pI serves as the first equivalence point of the amino acid.
At pH = 7.85, you will use pH = pKa2 + log([deprotonated]/[zwitterion]), because the pH > pI and the amino acid changes from 0 to -1 charge (so net negative charge).
At pH = 5.85, pH < pI and the amino acid changes from 0 to +1 charge (so net positive charge), so you will use pH = pKa1 + log([zwitterion]/[protonated]).
 rotonated and fully deprotonated:zwitterion?
rotonated and fully deprotonated:zwitterion?