Kaplan Section Test
Dehdyration reaction of secondary & tertiary alcohols is E1, and and E2 for primary alcohols.
How do you know which undergoes E1 & E2 ?
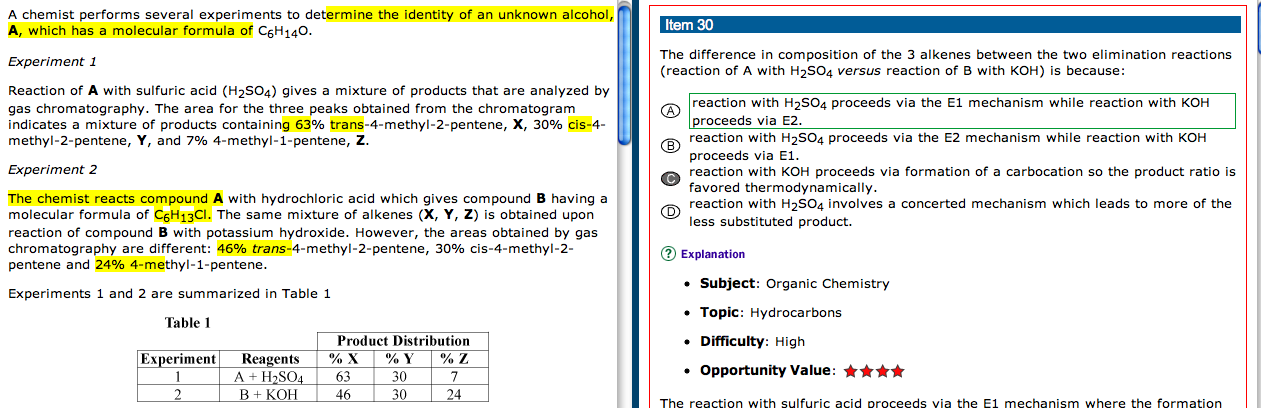
Dehdyration reaction of secondary & tertiary alcohols is E1, and and E2 for primary alcohols.
How do you know which undergoes E1 & E2 ?


 We are now watching the throne.
We are now watching the throne.