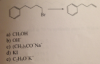
I feel like im right on this one..This is obviously E2 reaction with the adjacent carbon losing a hydrogen, but there are no other adjacent carbons other than that one. Do E2 reactions do rearrangements too?? The answer is the T-butyl (C), which I know is anti zaitsev in E2 reactions, but why would there be a need for anti zaitsev when there is only one adjacent carbon with hydrogens. Unless it rearranges to 2 carbons away somehow..? I felt the answer was OH-, the strong base and strong nucleophile that would result in E2 per usual.
Orgo, destroyer..??
- Thread starter JDHK
- Start date