D
DenTony11235
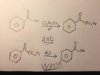
Whys this so??
Since RO(-) is better nucleophile than HO(-), wouldn't that make it a worse leaving group. So i get why the first reaction works, but why's the second one work...?
Does it mean that there would be an equilibrium going both ways? And if that's the case, would the equilibrium favor the formation of the ester?
Since RO(-) is better nucleophile than HO(-), wouldn't that make it a worse leaving group. So i get why the first reaction works, but why's the second one work...?
Does it mean that there would be an equilibrium going both ways? And if that's the case, would the equilibrium favor the formation of the ester?