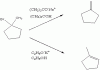- Joined
- May 16, 2008
- Messages
- 114
- Reaction score
- 0
Please explain the different reaction b/w these two...
I think these two reaction are E2. Why do they have different product?
Does the reaction follow the Zaltsev's rule?
I think these two reaction are E2. Why do they have different product?
Does the reaction follow the Zaltsev's rule?