- Joined
- Aug 7, 2015
- Messages
- 1,148
- Reaction score
- 859
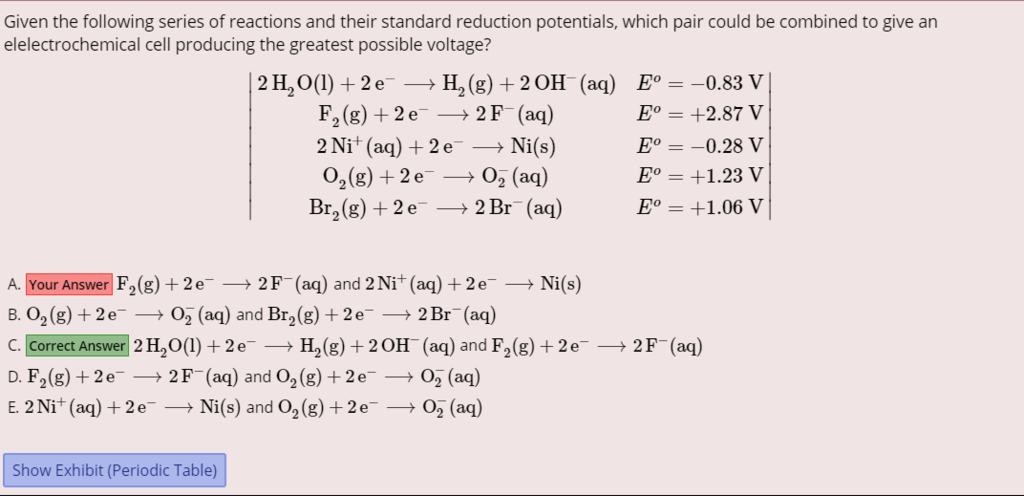
I need help on this Qvault question.
Don't you need am oxidation + reduction? How can you have a reduction + reduction as the highest potential?
Thanks!!
Don't you need am oxidation + reduction? How can you have a reduction + reduction as the highest potential?
Thanks!!
Last edited:
