- Joined
- Jun 27, 2004
- Messages
- 136
- Reaction score
- 0
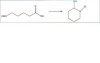
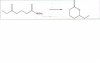
Hey everyone. In organic 2, I just finished a chapter on carboxylic acid derivatives. My professor gave us some practice problems to look at that he said would help us on the next test. One of these problems is similar to the intramolecular synthesis of a lactam, which my book seriously only has one paragraph on that is only a description of lactams in general. I was wondering if anyone could give me some info on how to do these? I've attached one of the sample problems, but I'm clueless how to go about doing it?