- Joined
- Jan 18, 2011
- Messages
- 84
- Reaction score
- 42
- Points
- 4,706
- Location
- Beyond the Wall
- Medical Student
The TL;DR version is in bold. Thanks!
ISSUE 1: According to the TBR Biology part 2 book (section 7 - Metabolic Pathways; p. 195 in the newest edition - copyrighted 2016), oxalosuccinate is a beta-keto acid. In fact, the TBR book doesn't even call it oxalosuccinate (or oxalosuccinic acid); they simply refer to the metabolite as "β-Keto acid". I thought it was an α-Keto acid. Is this a mistype?
Below is the Kreb cycle metabolite oxalosuccinate (though TBR depicted a fully unprotonated version in the book; I'm not sure if that makes any difference to the issues I have below). It looks like an alpha-keto acid. If it's actually not one, can someone explain or maybe pull up a picture/diagram that will clearly show how that is? I thought I knew the difference between the alpha and beta-keto acid forms. I guess not. Oxalosuccinate escapes me.
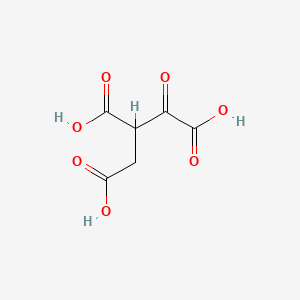
ISSUE 2: Upon further research (i.e. a 10-minute google search), I have noticed some inconsistent data. Some sources referred to oxalosuccinate as a β-keto acid while others (the majority, it seemed) referred to it as an α-keto acid. I would love some clarity on this.
ISSUE 3 : TBR explains that oxalosuccinate is unstable because of its beta-keto form, which results in a spontaneous decarboxylation. How, Sway? (And then proceeds to use a simple/generic chemical structure of a beta-keto acid - not oxalosuccinate itself, which, to me, does not resemble the generic beta-keto acid structure - to explain the decarboxylation mechanism 😕).
(And then proceeds to use a simple/generic chemical structure of a beta-keto acid - not oxalosuccinate itself, which, to me, does not resemble the generic beta-keto acid structure - to explain the decarboxylation mechanism 😕).
ISSUE 4: I'm having trouble understanding how an alpha-keto acid, in general, is more stable than a beta-keto acid. I'm thinking that the carbonyl groups being closer in proximity in an alpha-keto acid would make an alpha-keto acid less stable than a beta-keto acid. Please help me understand what I'm missing/getting wrong.
If anyone can shine some light into any of these issues, I would greatly appreciate it. One piece of information might jog my memory. 🙂
ISSUE 1: According to the TBR Biology part 2 book (section 7 - Metabolic Pathways; p. 195 in the newest edition - copyrighted 2016), oxalosuccinate is a beta-keto acid. In fact, the TBR book doesn't even call it oxalosuccinate (or oxalosuccinic acid); they simply refer to the metabolite as "β-Keto acid". I thought it was an α-Keto acid. Is this a mistype?
Below is the Kreb cycle metabolite oxalosuccinate (though TBR depicted a fully unprotonated version in the book; I'm not sure if that makes any difference to the issues I have below). It looks like an alpha-keto acid. If it's actually not one, can someone explain or maybe pull up a picture/diagram that will clearly show how that is? I thought I knew the difference between the alpha and beta-keto acid forms. I guess not. Oxalosuccinate escapes me.
ISSUE 2: Upon further research (i.e. a 10-minute google search), I have noticed some inconsistent data. Some sources referred to oxalosuccinate as a β-keto acid while others (the majority, it seemed) referred to it as an α-keto acid. I would love some clarity on this.
ISSUE 3 : TBR explains that oxalosuccinate is unstable because of its beta-keto form, which results in a spontaneous decarboxylation. How, Sway?
ISSUE 4: I'm having trouble understanding how an alpha-keto acid, in general, is more stable than a beta-keto acid. I'm thinking that the carbonyl groups being closer in proximity in an alpha-keto acid would make an alpha-keto acid less stable than a beta-keto acid. Please help me understand what I'm missing/getting wrong.
If anyone can shine some light into any of these issues, I would greatly appreciate it. One piece of information might jog my memory. 🙂
