- Joined
- Jun 24, 2014
- Messages
- 17
- Reaction score
- 0
Which three-dimensional conformation corresponds to the 3-hydroxy-cis-decalin, shown below?
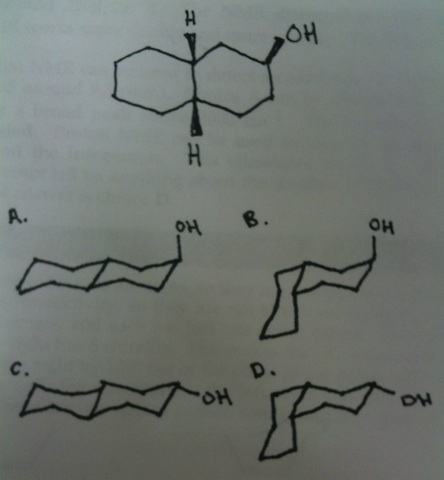
The hydroxyl group should be up, which allows us to eliminate C and D.
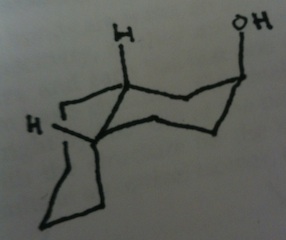
TBR says that the C-C bonds of the left ring must be one axial and one equatorial as a consequence of the hydrogens on the right ring being axial and equatorial. I don't follow their logic. Would the equatorial hydrogen on the first ring cause this orientation?

The hydroxyl group should be up, which allows us to eliminate C and D.

TBR says that the C-C bonds of the left ring must be one axial and one equatorial as a consequence of the hydrogens on the right ring being axial and equatorial. I don't follow their logic. Would the equatorial hydrogen on the first ring cause this orientation?
