- Joined
- Sep 28, 2016
- Messages
- 17
- Reaction score
- 1
The standard galvanic cell is constructed using Cr and Pt electrodes and 500 mL of the metal ion solutions in each half-cell. When a total of 6.0 g of metal is deposited at the cathode, what is the new associated concentration of the metal ion in the oxidation half-cell?
A. 0.64 M
B. 0.96 M
C. 1.04 M Correct Answer
D. 1.36 M
The Answer
The spontaneous reaction between Cr and Pt and their respective ions based on standard reduction potentials given in Table 1 is 2 Cr + 3 Pt2+ → 2 Cr3+ + 3 Pt, with an E°cell = 1.93 V. This indicates that Pt2+ is reduced at the cathode to deposit 6 g of Pt, and the Cr electrode is being oxidized to Cr3+ in the oxidation half-cell. This will increase [Cr3+] in the solution from 1 M, which is the concentration required for a standard cell (eliminate choices A and B).
To determine the new concentration, convert the mass of Pt into moles of Cr3+:
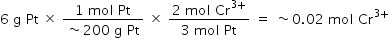
Five hundred milliliters of a 1 M solution will contain 0.5 mol Cr3+ to begin, so it will contain 0.52 mol in 0.5 L after the reaction indicated, making the new concentration 1.04 M (choice C is correct).
My question is I got lost when it said " This will increase [Cr3+] in the solution from 1 M, which is the concentration required for a standard cell" How do you know for sure that it will increase from 1 M? And also I get confused at their explanation afterwards as to how they arrive 1.04. Can someone kindly explain me in basic layman way lol. TPR is raping my brain cells T. T
A. 0.64 M
B. 0.96 M
C. 1.04 M Correct Answer
D. 1.36 M
The Answer
The spontaneous reaction between Cr and Pt and their respective ions based on standard reduction potentials given in Table 1 is 2 Cr + 3 Pt2+ → 2 Cr3+ + 3 Pt, with an E°cell = 1.93 V. This indicates that Pt2+ is reduced at the cathode to deposit 6 g of Pt, and the Cr electrode is being oxidized to Cr3+ in the oxidation half-cell. This will increase [Cr3+] in the solution from 1 M, which is the concentration required for a standard cell (eliminate choices A and B).
To determine the new concentration, convert the mass of Pt into moles of Cr3+:
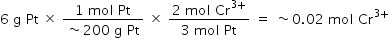
Five hundred milliliters of a 1 M solution will contain 0.5 mol Cr3+ to begin, so it will contain 0.52 mol in 0.5 L after the reaction indicated, making the new concentration 1.04 M (choice C is correct).
My question is I got lost when it said " This will increase [Cr3+] in the solution from 1 M, which is the concentration required for a standard cell" How do you know for sure that it will increase from 1 M? And also I get confused at their explanation afterwards as to how they arrive 1.04. Can someone kindly explain me in basic layman way lol. TPR is raping my brain cells T. T
