- Joined
- Dec 25, 2010
- Messages
- 2,340
- Reaction score
- 17
In the reaction shown in equation 1, Al(OH)3 acts as what kind of acid or base?
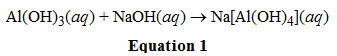
A. Lewis Acid
B. Lewis Base
C. Bronsted Acid
D. Bronsted Base
Answer: A
I picked A because just from prior knowledge I know that for these types of compounds the metal almost always acts as a lewis acid. If I didn't know this from prior knowledge, how exactly do I know that what is accepting/donating electrons/protons?
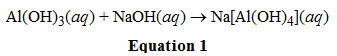
A. Lewis Acid
B. Lewis Base
C. Bronsted Acid
D. Bronsted Base
Answer: A
I picked A because just from prior knowledge I know that for these types of compounds the metal almost always acts as a lewis acid. If I didn't know this from prior knowledge, how exactly do I know that what is accepting/donating electrons/protons?

