- Joined
- Dec 25, 2010
- Messages
- 2,338
- Reaction score
- 17
- Points
- 4,641
- Medical Student
In the reaction shown in equation 1, Al(OH)3 acts as what kind of acid or base?
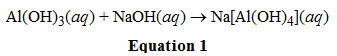
A. Lewis Acid
B. Lewis Base
C. Bronsted Acid
D. Bronsted Base
Answer: A
I picked A because just from prior knowledge I know that for these types of compounds the metal almost always acts as a lewis acid. If I didn't know this from prior knowledge, how exactly do I know that what is accepting/donating electrons/protons?
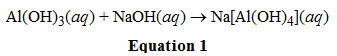
A. Lewis Acid
B. Lewis Base
C. Bronsted Acid
D. Bronsted Base
Answer: A
I picked A because just from prior knowledge I know that for these types of compounds the metal almost always acts as a lewis acid. If I didn't know this from prior knowledge, how exactly do I know that what is accepting/donating electrons/protons?

