- Joined
- Mar 22, 2016
- Messages
- 254
- Reaction score
- 975
Hello! So this is something that I've stumbled across a few times, and it seems like the ranking convention might change based on whatever review material I'm using. When assigning stereochemistry to a "complex" stereocenter where there are multiple heteroatoms, how does the AAMC want us to rank the substituents? An example is below.
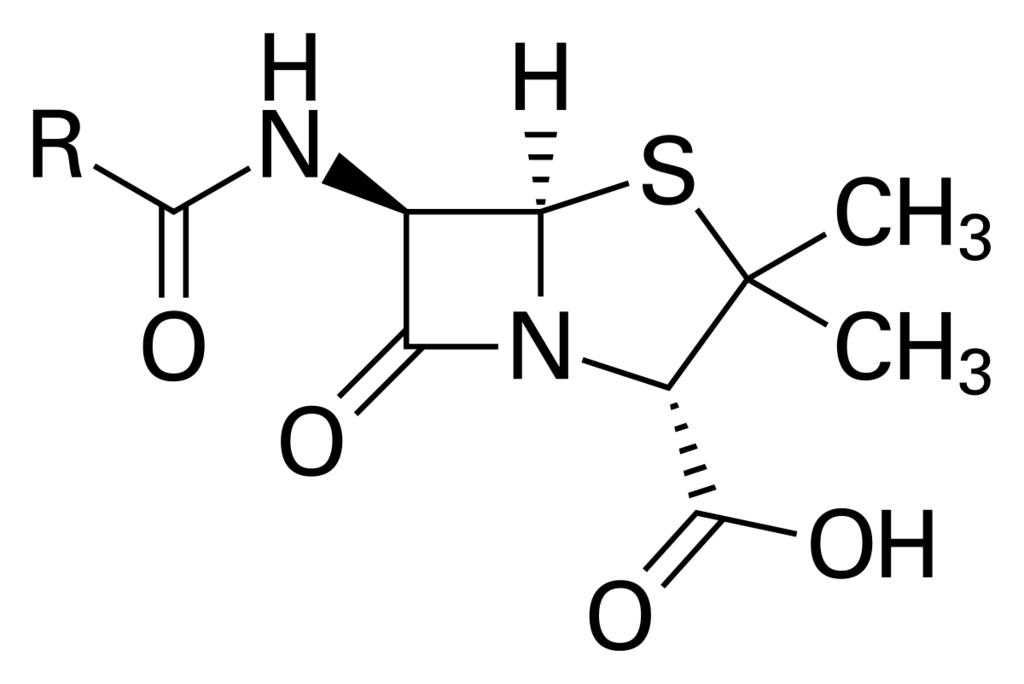
When looking at the bottom right stereocenter (C connected to N, C, C), I assigned the stereochemistry to be S. TBR tells me that it is R.
Keeping the H in the front, I assigned the substituents as follows:
1 = N, because N>C
2 = C - (S, C, C) because S > O in the next substituent
3 = C - (O, O, O) ranked as lowest because S > O, and I was taught that the first difference is the one you rank around. Because S has a higher atomic number than O, I ranked C-S > C-O
Also, as a side note - TBR correctly (in my eyes) ranks the top left stereocenter as R, following this same idea - N beats C (connected to S, N, H) beats C (connected to O, O, N)
Is this a lapse in my misunderstanding or is it just a funky, specific convention that isn't followed sometimes? I remember encountering this a year or two back as well, but we didn't reach a conclusion. What do you all think?
Thank you!!
When looking at the bottom right stereocenter (C connected to N, C, C), I assigned the stereochemistry to be S. TBR tells me that it is R.
Keeping the H in the front, I assigned the substituents as follows:
1 = N, because N>C
2 = C - (S, C, C) because S > O in the next substituent
3 = C - (O, O, O) ranked as lowest because S > O, and I was taught that the first difference is the one you rank around. Because S has a higher atomic number than O, I ranked C-S > C-O
Also, as a side note - TBR correctly (in my eyes) ranks the top left stereocenter as R, following this same idea - N beats C (connected to S, N, H) beats C (connected to O, O, N)
Is this a lapse in my misunderstanding or is it just a funky, specific convention that isn't followed sometimes? I remember encountering this a year or two back as well, but we didn't reach a conclusion. What do you all think?
Thank you!!

