D
deleted647690
I got the correct answer, but I want to make sure my reasoning was okay. I decided it couldn't be B because a disaccharide can't be reducing if both anomeric carbons are tied up in the glycosidic linkage. For the same reason, you can cross off C because it would be reducing if only one anomeric carbon was tied up in the linkage.
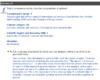
Both A and D are accurate in their descriptions of reducing and non reducing sugars, but I only chose A because I knew that maltose was made up of two glucose sugars (from the passage), and I knew glucose was reducing, so I kind of just guessed.
EDIT: Actually, I just realized it told you in the passage it was a 1--->4 bond, so that tells you the answer since you know that in glucose, carbon 1 is the anomeric carbon
Both A and D are accurate in their descriptions of reducing and non reducing sugars, but I only chose A because I knew that maltose was made up of two glucose sugars (from the passage), and I knew glucose was reducing, so I kind of just guessed.
EDIT: Actually, I just realized it told you in the passage it was a 1--->4 bond, so that tells you the answer since you know that in glucose, carbon 1 is the anomeric carbon