- Joined
- Nov 9, 2006
- Messages
- 110
- Reaction score
- 0
one of question in achiever test 1 in ochem part was
which of conformation of cyclohexane with 3 methyl group (1,2,4) attached are stable.
and answer was the three methyl group all on axial position
but if 1and 2 are both equitorial position, isn't the steric hindrance greater than 1 being equitorial and 2 being axial?
because if 2 is in axial the only steric hindrance is from H from 3 which is better than CH3 from 1 if 2 is in equitorial position..
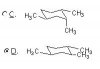
to me C is less sterically hindered than d but answer was d..
whyyyy..
which of conformation of cyclohexane with 3 methyl group (1,2,4) attached are stable.
and answer was the three methyl group all on axial position
but if 1and 2 are both equitorial position, isn't the steric hindrance greater than 1 being equitorial and 2 being axial?
because if 2 is in axial the only steric hindrance is from H from 3 which is better than CH3 from 1 if 2 is in equitorial position..

to me C is less sterically hindered than d but answer was d..
whyyyy..

